Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation
- PMID: 18957049
- PMCID: PMC2586876
- DOI: 10.1111/j.1399-3089.2008.00480.x
Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation
Abstract
Background: Experience with non-antigenic galactose alpha1,3 galactose (alphaGal) polymers and development of alphaGal deficient pigs has reduced or eliminated the significance of this antigen in xenograft rejection. Despite these advances, delayed xenograft rejection (DXR) continues to occur most likely due to antibody responses to non-Gal endothelial cell (EC) antigens.
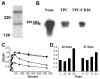
Methods: To gauge the diversity of the non-Gal antibody response we used antibody derived from CD46 transgenic heterotopic cardiac xenografts performed without T-cell immunosuppression, Group A (n = 4) and Gal knockout (GT-KO) heart transplants under tacrolimus and sirolimus immunosuppression, Group B (n = 8). Non-Gal antibody was measured by flow cytometry and by western blots using GT-KO EC membrane antigens. A nanoLC/MS/MS analysis of proteins recovered from 2D gels was used to identify target antigens.
Results: Group A recipients exhibited a mixed cellular and humoral rejection. Group B recipients mainly exhibited classical DXR. Western blot analysis showed a non-Gal antibody response induced by GT+ and GT-KO hearts to an overlapping set of pig aortic EC membrane antigens. Proteomic analysis identified 14 potential target antigens but failed to define several immunodominant targets.
Conclusions: These experiments indicate that the non-Gal antibody response is directed to a number of stress response and inflammation related pig EC antigens and a few undefined targets. Further analysis of these antibody specificities using alternative methods is required to more fully define the repertoire of non-Gal antibody responses.
Figures



References
-
- LEVENTHAL JR, SAKIYALAK P, WITSON J, et al. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1993;57:974–978. - PubMed
-
- LIN SS, KOOYMAN DL, DANIELS LJ, et al. The role of natural anti-Gal-alpha-1-3Gal antibodies in hyperacute rejection of pig-to-baboon cardiac xenotransplants. Transplant Immunol. 1997;5:212–218. - PubMed
-
- LOGAN JS. Prospects for xenotransplantation. Curr Opin Immunol. 2000;12:563–568. - PubMed
-
- DORLING A. Are anti-endothelial cell antibodies a pre-requisite for the acute vascular rejection of xenografts? Xenotransplantation. 2003;10:16–23. - PubMed
-
- MCGREGOR CGA, TEOTIA SS, BYRNE GW, et al. Cardiac xenotransplantation: Progress toward the clinic. Transplantation. 2004;78:1569–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

