Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation
- PMID: 18957053
- PMCID: PMC2857931
- DOI: 10.1111/j.1471-4159.2008.05708.x
Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation
Abstract
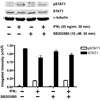
The proinflammatory cytokine interferon-gamma (IFNgamma) alters neuronal connectivity via selective regressive effects on dendrites but the signaling pathways that mediate this effect are poorly understood. We recently demonstrated that signaling by Rit, a member of the Ras family of GTPases, modulates dendritic growth in primary cultures of sympathetic and hippocampal neurons. In this study, we investigated a role for Rit signaling in IFNgamma-induced dendritic retraction. Expression of a dominant negative Rit mutant inhibited IFNgamma-induced dendritic retraction in cultured embryonic rat sympathetic and hippocampal neurons. In pheochromacytoma cells and hippocampal neurons, IFNgamma caused rapid Rit activation as indicated by increased GTP binding to Rit. Silencing of Rit by RNA interference suppressed IFNgamma-elicited activation of p38 MAPK in pheochromacytoma cells, and pharmacological inhibition of p38 MAPK significantly attenuated the dendrite-inhibiting effects of IFNgamma in cultured sympathetic and hippocampal neurons without altering signal transducer and activator of transcription 1 activation. These observations identify Rit as a downstream target of IFNgamma and suggest that a novel IFNgamma-Rit-p38 signaling pathway contributes to dendritic retraction and may, therefore, represent a potential therapeutic target in diseases with a significant neuroinflammatory component.
Figures





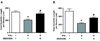
References
-
- Arendt T, Gartner U, Seeger G, Barmashenko G, Palm K, Mittmann T, Yan L, Hummeke M, Behrbohm J, Bruckner MK, Holzer M, Wahle P, Heumann R. Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci. 2004;19:2953–2966. - PubMed
-
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. - PubMed
-
- Binder GK, Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. - PubMed
-
- Brannstrom T, Havton L, Kellerth JO. Changes in size and dendritic arborization patterns of adult cat spinal alpha-motoneurons following permanent axotomy. J Comp Neurol. 1992;318:439–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

