A feasibility study of immediate versus deferred antiretroviral therapy in children with HIV infection
- PMID: 18957095
- PMCID: PMC2584102
- DOI: 10.1186/1742-6405-5-24
A feasibility study of immediate versus deferred antiretroviral therapy in children with HIV infection
Abstract
Objective: To evaluate the feasibility of a large immediate versus deferred antiretroviral therapy (ART) study in children.
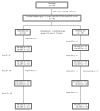
Methods: We conducted an open-label pilot randomized clinical trial study in 43 Thai children with CD4 15 to 24% of starting generic AZT/3TC/NVP immediately (Arm 1) or deferring until CD4 < 15% or CDC C (Arm 2). Primary endpoints were recruitment rate, adherence to randomized treatment and retention in trial. Secondary endpoints were % with CDC C or CD4 < 15%. Children were in the trial until the last child reached 108 weeks. Intention to treat and on treatment analyses were performed.
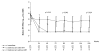
Results: Recruitment took 15 months. Twenty-six of 69 (37.7%) were not eligible due mainly to low CD4%. Twenty four and 19 were randomized to arms 1 and 2 respectively. All accepted the randomized arm; however, 3 in arm 1 stopped ART and 1 in arm 2 refused to start ART. Ten/19 (53%) in arm 2 started ART. At baseline, median age was 4.8 yrs, CDC A:B were 36:7, median CD4 was 19% and viral load was 4.8 log. All in arm 1 and 17/19 in arm 2 completed the study (median of 134 weeks). No one had AIDS or death. Four in immediate arm had tuberculosis. Once started on ART, deferred arm children achieved similar CD4 and viral load response as the immediate arm. Adverse events were similar between arms. The deferred arm had a 26% ART saving.
Conclusion: Almost 40% of children were not eligible due mainly to low CD4% but adherence to randomized treatment and retention in trial were excellent. A larger study to evaluate when to start ART is feasible.
Figures

References
-
- Violari A, Cotton M, Gibb D, Babiker A, Steyn J, Jean-Phillip P, McIntyre J, Team obotCS. Antiretroviral therapy initiated before 12 weeks of age reduces early mortality in young HIV-infected infants: evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study [Abstract WESS 103] 4th IAS Conference on HIV Pathogenesis and Treatment; Sydney, July 22–25, 2007.
-
- McConnell MS, Byers RH, Frederick T, Peters VB, Dominguez KL, Sukalac T, Greenberg AE, Hsu HW, Rakusan TA, Ortiz IR, Melville SK, Fowler MG. Trends in antiretroviral therapy use and survival rates for a large cohort of HIV-infected children and adolescents in the United States, 1989–2001. J Acquir Immune Defic Syndr. 2005;38:488–494. doi: 10.1097/01.qai.0000134744.72079.cc. - DOI - PubMed
-
- Doerholt K, Duong T, Tookey P, Butler K, Lyall H, Sharland M, Novelli V, Riordan A, Dunn D, Walker AS, Gibb DM. Outcomes for human immunodeficiency virus-1-infected infants in the United kingdom and Republic of Ireland in the era of effective antiretroviral therapy. Pediatr Infect Dis J. 2006;25:420–426. doi: 10.1097/01.inf.0000214994.44346.d3. - DOI - PubMed
-
- LePrevost M, Green H, Flynn J, Head S, Clapson M, Lyall H, Novelli V, Farrelly L, Walker AS, Burger DM, Gibb DM. Adherence and acceptability of once daily Lamivudine and abacavir in human immunodeficiency virus type-1 infected children. Pediatr Infect Dis J. 2006;25:533–537. doi: 10.1097/01.inf.0000222415.40563.d4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

