Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity
- PMID: 18957222
- PMCID: PMC2634746
- DOI: 10.1016/j.neuron.2008.08.012
Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity
Abstract
Normal brain function requires that the overall synaptic activity in neural circuits be kept constant. Long-term alterations of neural activity lead to homeostatic regulation of synaptic strength by a process known as synaptic scaling. The molecular mechanisms underlying synaptic scaling are largely unknown. Here, we report that all-trans retinoic acid (RA), a well-known developmental morphogen, unexpectedly mediates synaptic scaling in response to activity blockade. We show that activity blockade increases RA synthesis in neurons and that acute RA treatment enhances synaptic transmission. The RA-induced increase in synaptic strength is occluded by activity blockade-induced synaptic scaling. Suppression of RA synthesis prevents synaptic scaling. This form of RA signaling operates via a translation-dependent but transcription-independent mechanism, causes an upregulation of postsynaptic glutamate receptor levels, and requires RARalpha receptors. Together, our data suggest that RA functions in homeostatic plasticity as a signaling molecule that increases synaptic strength by a protein synthesis-dependent mechanism.
Figures

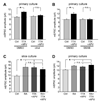
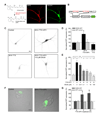
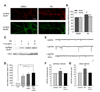
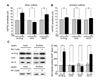
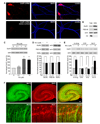
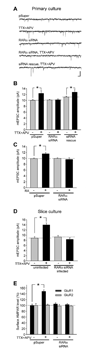

Comment in
-
A role for retinoic acid in homeostatic plasticity.Neuron. 2008 Oct 23;60(2):192-4. doi: 10.1016/j.neuron.2008.10.003. Neuron. 2008. PMID: 18957211 Free PMC article.
References
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
-
- Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol Rev. 1991;71:951–990. - PubMed
-
- Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. Faseb J. 2008;22:236–245. - PubMed
-
- Chiang MY, Misner D, Kempermann G, Schikorski T, Giguere V, Sucov HM, Gage FH, Stevens CF, Evans RM. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 1998;21:1353–1361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

