Reverberation of recent visual experience in spontaneous cortical waves
- PMID: 18957223
- PMCID: PMC3576032
- DOI: 10.1016/j.neuron.2008.08.026
Reverberation of recent visual experience in spontaneous cortical waves
Abstract
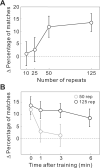
Spontaneous waves of activity propagating across large cortical areas may play important roles in sensory processing and circuit refinement. However, whether these waves are in turn shaped by sensory experience remains unclear. Here we report that visually evoked cortical activity reverberates in subsequent spontaneous waves. Voltage-sensitive dye imaging in rat visual cortex shows that following repetitive presentation of a given visual stimulus, spatiotemporal activity patterns resembling the evoked response appear more frequently in the spontaneous waves. This effect is specific to the response pattern evoked by the repeated stimulus, and it persists for several minutes without further visual stimulation. Such wave-mediated reverberation could contribute to short-term memory and help to consolidate the transient effects of recent sensory experience into long-lasting cortical modifications.
Figures




References
-
- Abeles M, Gerstein GL. Detecting spatiotemporal firing patterns among simultaneously recorded single neurons. J Neurophysiol. 1988;60:909–924. - PubMed
-
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol. 1995;73:20–38. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Averbach E, Sperling G. Short term storage of information in vision. In: Cherry C, editor. Information Theory. Butterworth; London: 1961. pp. 196–211.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

