Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events
- PMID: 18957228
- PMCID: PMC2628634
- DOI: 10.1016/j.neuron.2008.09.023
Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events
Abstract
Decisions are often guided by generalizing from past experiences. Fundamental questions remain regarding the cognitive and neural mechanisms by which generalization takes place. Prior data suggest that generalization may stem from inference-based processes at the time of generalization. By contrast, generalization may emerge from mnemonic processes occurring while premise events are encoded. Here, participants engaged in a two-phase learning and generalization task, wherein they learned a series of overlapping associations and subsequently generalized what they learned to novel stimulus combinations. Functional MRI revealed that successful generalization was associated with coupled changes in learning-phase activity in the hippocampus and midbrain (ventral tegmental area/substantia nigra). These findings provide evidence for generalization based on integrative encoding, whereby overlapping past events are integrated into a linked mnemonic representation. Hippocampal-midbrain interactions support the dynamic integration of experiences, providing a powerful mechanism for building a rich associative history that extends beyond individual events.
Figures

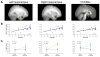
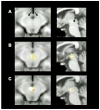
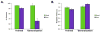


Comment in
-
The hippocampus and dopaminergic midbrain: old couple, new insights.Neuron. 2008 Oct 23;60(2):197-200. doi: 10.1016/j.neuron.2008.10.007. Neuron. 2008. PMID: 18957213
References
-
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. - PubMed
-
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J Neurophysiol. 2004;92:1144–1152. - PubMed
-
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. - PubMed
-
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

