Enhanced activity of human serotonin transporter variants associated with autism
- PMID: 18957375
- PMCID: PMC2674096
- DOI: 10.1098/rstb.2008.0143
Enhanced activity of human serotonin transporter variants associated with autism
Abstract
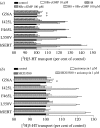
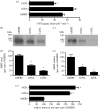
Rare, functional, non-synonymous variants in the human serotonin (5-hydroxytryptamine, 5-HT) transporter (hSERT) gene (SLC6A4) have been identified in both autism and obsessive-compulsive disorder (OCD). Within autism, rare hSERT coding variants associate with rigid-compulsive traits, suggesting both phenotypic overlap with OCD and a shared relationship with disrupted 5-HT signalling. Here, we document functional perturbations of three of these variants: Ile425Leu; Phe465Leu; and Leu550Val. In transiently transfected HeLa cells, the three variants confer a gain of 5-HT transport phenotype. Specifically, enhanced SERT activity was also observed in lymphoblastoid lines derived from mutation carriers. In contrast to previously characterized Gly56Ala, where increased transport activity derives from catalytic activation, the three novel variants exhibit elevated surface density as revealed through both surface antagonist-binding and biotinylation studies. Unlike Gly56Ala, mutants Ile425Leu, Phe465Leu and Leu550Val retain a capacity for acute PKG and p38 MAPK regulation. However, both Gly56Ala and Ile425Leu demonstrate markedly reduced sensitivity to PP2A antagonists, suggesting that deficits in trafficking and catalytic modulation may derive from a common basis in perturbed phosphatase regulation. When expressed stably from the same genomic locus in CHO cells, both Gly56Ala and Ile425Leu display catalytic activation, accompanied by a striking loss of SERT protein.
Figures



References
-
- Altamura C., Dell'Acqua M.L., Moessner R., Murphy D.L., Lesch K.P., Persico A.M. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb. Cortex. 2007;17:1394–1401. doi:10.1093/cercor/bhl051 - DOI - PubMed
-
- Balkovetz D.F., Tiruppathi C., Leibach F.H., Mahesh V.B., Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J. Biol. Chem. 1989;264:2195–2198. - PubMed
-
- Bengel D., Murphy D.L., Andrews A.M., Wichems C.H., Feltner D., Heils A., Mossner R., Westphal H., Lesch K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymetamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998;53:649–655. - PubMed
-
- Blakely R.D., Bauman A.L. Biogenic amine transporters: regulation in flux. Curr. Opin. Neurobiol. 2000;10:328–336. doi:10.1016/S0959-4388(00)00088-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
