Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin
- PMID: 18957474
- PMCID: PMC2644651
- DOI: 10.1093/hmg/ddn337
Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin
Abstract
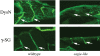
Sapje-like (sap(cl100)) was one of eight potential zebrafish muscle mutants isolated as part of an early-pressure screen of 500 families. This mutant shows a muscle tearing phenotype similar to sapje (dys-/-) and both mutants fail to genetically complement suggesting they have a mutation in the same gene. Protein analysis confirms a lack of dystrophin in developing sapje-like embryos. Sequence analysis of the sapje-like dystrophin mRNA shows that exon 62 is missing in the dystrophin transcript causing exon 63 to be translated out of frame terminating translation at a premature stop codon at the end of exon 63. Sequence analysis of sapje-like genomic DNA identified a mutation in the donor splice junction at the end of dystrophin exon 62. This mutation is similar to splicing mutations associated with human forms of Duchenne Muscular Dystrophy. Sapje-like is the first zebrafish dystrophin splicing mutant identified to date and represents a novel disease model which can be used in future studies to identify therapeutic compounds for treating diseases caused by splicing defects.
Figures




References
-
- Burghes A.H., Logan C., Hu X., Belfall B., Worton R.G., Ray P.N. A cDNA clone from the Duchenne/Becker muscular dystrophy gene. Nature. 1987;328:434–437. - PubMed
-
- Hoffman E.P., Brown R.H., Jr, Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Monaco A.P., Neve R.L., Colletti-Feener C., Bertelson C.J., Kurnit D.M., Kunkel L.M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. - PubMed
-
- Hoffman E.P., Fischbeck K.H., Brown R.H., Johnson M., Medori R., Loike J.D., Harris J.B., Waterston R., Brooke M., Specht L., et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N. Engl. J. Med. 1988;318:1363–1368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

