Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating
- PMID: 18957515
- PMCID: PMC2740334
- DOI: 10.1242/jcs.029678
Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating
Abstract
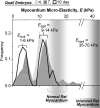
Fibrotic rigidification following a myocardial infarct is known to impair cardiac output, and it is also known that cardiomyocytes on rigid culture substrates show a progressive loss of rhythmic beating. Here, isolated embryonic cardiomyocytes cultured on a series of flexible substrates show that matrices that mimic the elasticity of the developing myocardial microenvironment are optimal for transmitting contractile work to the matrix and for promoting actomyosin striation and 1-Hz beating. On hard matrices that mechanically mimic a post-infarct fibrotic scar, cells overstrain themselves, lack striated myofibrils and stop beating; on very soft matrices, cells preserve contractile beating for days in culture but do very little work. Optimal matrix leads to a strain match between cell and matrix, and suggests dynamic differences in intracellular protein structures. A 'cysteine shotgun' method of labeling the in situ proteome reveals differences in assembly or conformation of several abundant cytoskeletal proteins, including vimentin, filamin and myosin. Combined with recent results, which show that stem cell differentiation is also highly sensitive to matrix elasticity, the methods and analyses might be useful in the culture and assessment of cardiogenesis of both embryonic stem cells and induced pluripotent stem cells. The results described here also highlight the need for greater attention to fibrosis and mechanical microenvironments in cell therapy and development.
Figures





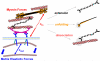
References
-
- Barry CP, Xie J, Lemmon V, Young AP. Molecular characterization of a multi-promoter gene encoding a chicken filamin protein. J. Biol. Chem. 1993;268:25577–25586. - PubMed
-
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2196–H2203. - PubMed
-
- Bird SD, Doevendans PA, van Rooijen MA, Brutel de la Riviere A, Hassink RJ, Passier R, Mummery CL. The human adult cardiomyocyte phenotype. Cardiovasc. Res. 2003;58:423–434. - PubMed
-
- Birks E, Hall J, Barton P, Grindle S, Latif N, Hardy J, Rider J, Banner N, Khaghani A, Miller L, et al. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112:157–164. - PubMed
-
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

