Antitumor efficacy testing in rodents
- PMID: 18957675
- PMCID: PMC2597219
- DOI: 10.1093/jnci/djn351
Antitumor efficacy testing in rodents
Abstract
The preclinical research and human clinical trials necessary for developing anticancer therapeutics are costly. One contributor to these costs is preclinical rodent efficacy studies, which, in addition to the costs associated with conducting them, often guide the selection of agents for clinical development. If inappropriate or inaccurate recommendations are made on the basis of these preclinical studies, then additional costs are incurred. In this commentary, I discuss the issues associated with preclinical rodent efficacy studies. These include the identification of proper preclinical efficacy models, the selection of appropriate experimental endpoints, and the correct statistical evaluation of the resulting data. I also describe important experimental design considerations, such as selecting the drug vehicle, optimizing the therapeutic treatment plan, properly powering the experiment by defining appropriate numbers of replicates in each treatment arm, and proper randomization. Improved preclinical selection criteria can aid in reducing unnecessary human studies, thus reducing the overall costs of anticancer drug development.
Figures
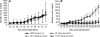
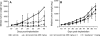


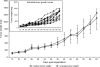
Comment in
-
Re: Antitumor efficacy testing in rodents.J Natl Cancer Inst. 2009 Nov 18;101(22):1592-3; author reply 1593-4. doi: 10.1093/jnci/djp356. Epub 2009 Oct 28. J Natl Cancer Inst. 2009. PMID: 19864637 No abstract available.
References
-
- American Cancer Society. Cancer Facts and Figures 2008. Atlanta, GA: American Cancer Society; 2008.
-
- Venkatesh S, Lipper RA. Role of the development scientist in compound lead selection and optimization. J Pharm Sci. 2000;89(2):145–154. - PubMed
-
- Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. J Clin Oncol. 2003;21(22):4145–4150. - PubMed
-
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–185. - PubMed
-
- Hermiston TW, Kirn DH. Genetically based therapeutics for cancer: similarities and contrasts with traditional drug discovery and development. Mol Ther. 2005;11(4):496–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

