Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy
- PMID: 18957684
- PMCID: PMC2630266
- DOI: 10.1182/blood-2008-10-181479
Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy
Abstract
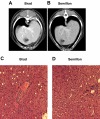
Preclinical studies and initial clinical trials have documented the feasibility of adenoassociated virus (AAV)-mediated gene therapy for hemophilia B. In an 8-year study, inhibitor-prone hemophilia B dogs (n = 2) treated with liver-directed AAV2 factor IX (FIX) gene therapy did not have a single bleed requiring FIX replacement, whereas dogs undergoing muscle-directed gene therapy (n = 3) had a bleed frequency similar to untreated FIX-deficient dogs. Coagulation tests (whole blood clotting time [WBCT], activated clotting time [ACT], and activated partial thromboplastin time [aPTT]) have remained at the upper limits of the normal ranges in the 2 dogs that received liver-directed gene therapy. The FIX activity has remained stable between 4% and 10% in both liver-treated dogs, but is undetectable in the dogs undergoing muscle-directed gene transfer. Integration site analysis by linear amplification-mediated polymerase chain reaction (LAM-PCR) suggested the vector sequences have persisted predominantly in extrachromosomal form. Complete blood count (CBC), serum chemistries, bile acid profile, hepatic magnetic resonance imaging (MRI) and computed tomography (CT) scans, and liver biopsy were normal with no evidence for tumor formation. AAV-mediated liver-directed gene therapy corrected the hemophilia phenotype without toxicity or inhibitor development in the inhibitor-prone null mutation dogs for more than 8 years.
Figures
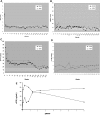
 ). Normal ranges of 1 to 2 minutes for ACT, 6 to 10 minutes for WBCT, and 8.0 to 14.4 seconds for aPTT are indicated by the shaded bars.
). Normal ranges of 1 to 2 minutes for ACT, 6 to 10 minutes for WBCT, and 8.0 to 14.4 seconds for aPTT are indicated by the shaded bars.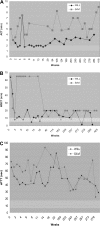
 ). The arrows indicate cyclophosphamide administration to Wilbur before and 2, 4, and 6 weeks after vector administration. The WBCT test was terminated at 60 minutes if a clot had not formed for the first 15 weeks. After 15 weeks, the WBCT test was terminated after 20 minutes if a clot had not formed.
). The arrows indicate cyclophosphamide administration to Wilbur before and 2, 4, and 6 weeks after vector administration. The WBCT test was terminated at 60 minutes if a clot had not formed for the first 15 weeks. After 15 weeks, the WBCT test was terminated after 20 minutes if a clot had not formed.



References
-
- Rawle FE, Lillicrap D. Preclinical animal models for hemophilia gene therapy: predictive value and limitations. Semin Thromb Hemost. 2004;30:205–213. - PubMed
-
- Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. - PubMed
-
- Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. - PubMed
-
- Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. - PubMed
-
- High KA. Gene transfer as an approach to treating hemophilia. Semin Thromb Hemost. 2003;29:107–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

