Isoprostanes
- PMID: 18957694
- PMCID: PMC2674741
- DOI: 10.1194/jlr.R800037-JLR200
Isoprostanes
Abstract
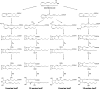
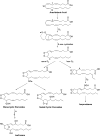
The isoprostanes (IsoPs) are a unique series of prostaglandin-like compounds formed in vivo via a nonenzymatic mechanism involving the free radical-initiated peroxidation of arachidonic acid. This article summarizes our current knowledge of these compounds. Herein, a historical account of their discovery and the mechanism of their formation are described. A specific class of IsoPs, the F2-IsoPs, are stable, robust molecules that can be measured as indices of endogenous oxidant stress. The utility of these molecules as biomarkers and methods by which these compounds can be quantified are discussed. In addition to the F2-IsoPs, isoprostanes with other prostane ring structures as well as oxidation products with furan and dioxolane rings can be generated from arachidonic acid. And, in more recent years, isoprostane-like compounds have been shown to be formed from polyunsaturated fatty acids including eicosapentaenoic acid [C20:5, omega-3], docosahexaenoic acid [C22:6, omega-3], and adrenic acid [C22:4, omega-6]. These findings will be summarized as well.
Figures
References
-
- Halliwell B., and J. M. Gutteridge. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186 1–85. - PubMed
-
- Kadiiska M. B., B. C. Gladen, D. D. Baird, D. Germolec, L. B. Graham, C. E. Parker, A. Nyska, J. T. Wachsman, B. N. Ames, S. Basu, et al. 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 38 698–710. - PubMed
-
- Fam S. S., and J. D. Morrow. 2003. The isoprostanes: unique products of arachidonic acid oxidation–a review. Curr. Med. Chem. 10 1723–1740. - PubMed
-
- Gao L., H. Yin, G. L. Milne, N. A. Porter, and J. D. Morrow. 2006. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J. Biol. Chem. 281 14092–14099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous