Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM
- PMID: 18957964
- PMCID: PMC2834981
- DOI: 10.1038/mt.2008.228
Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM
Abstract
We have previously demonstrated safety of G207, a doubly mutated (deletion of both gamma(1)34.5 loci, insertional inactivation of U(L)39) herpes simplex virus (HSV) for patients stereotactically inoculated in enhancing portions of recurrent malignant gliomas. We have now determined safety of two inoculations of G207, before and after tumor resection. Inclusion criteria were histologically proven recurrent malignant glioma, Karnofsky score >or=70, and ability to resect the tumor without ventricular system breach. Patients received two doses of G207 totaling 1.15 x 10(9) plaque-forming units with 13% of this total injected via a catheter placed stereotactically in the tumor. Two or five days later, tumor was resected en bloc with catheter in place. The balance of G207 dose was injected into brain surrounding the resection cavity. Six patients with recurrent glioblastoma multiforme were enrolled. Two days after the second G207 inoculation, one patient experienced transient fever, delirium, and hemiparesis, which entirely resolved on high-dose dexamethasone. No patient developed HSV encephalitis or required treatment with acyclovir. Radiographic and neuropathologic evidence suggestive of antitumor activity is reported. Evidence of viral replication was demonstrated. G207 appears safe for multiple dose delivery, including direct inoculation into the brain surrounding tumor resection cavity.
Figures
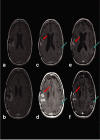
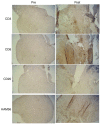

Comment in
-
Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication.Mol Ther. 2009 Jan;17(1):8-9. doi: 10.1038/mt.2008.275. Mol Ther. 2009. PMID: 19116635 Free PMC article. No abstract available.
References
-
- Chang SM, Butowski NA, Sneed PK., and , Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme. Neurosurg Focus. 2006;20:E4. - PubMed
-
- Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M., and , Schiff D.Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series Neurosurgery 19994517, 22–22.discussion - PubMed
-
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. - PubMed
-
- Chamberlain MC., and , Kormanik P. Salvage chemotherapy with taxol for recurrent anaplastic astrocytomas. J Neurooncol. 1999;43:71–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

