Auditory hallucinations in schizophrenia: the role of cognitive, brain structural and genetic disturbances in the left temporal lobe
- PMID: 18958220
- PMCID: PMC2525988
- DOI: 10.3389/neuro.09.006.2007
Auditory hallucinations in schizophrenia: the role of cognitive, brain structural and genetic disturbances in the left temporal lobe
Abstract
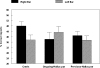
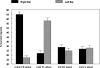
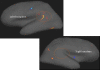
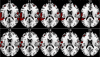
In this article we review research in our laboratory on auditory hallucinations using behavioral and MRI measure. The review consists of both previously published and new data that for the first time is presented together in a cohesive way. Auditory hallucinations are among the most common symptoms in schizophrenia, affecting more than 70% of the patients. We here advance the hypothesis that auditory hallucinations are internally generated speech perceptions that are lateralized to the left temporal lobe, in the peri-Sylvian region. From this we predict that hallucinating patients should have problems identifying a simultaneously presented external speech sound, as measured through performance on the dichotic listening (DL) paradigm with consonant-vowel syllables, since this technique lateralizes the stimulus input. Across a series of behavioral experiments, we have shown that patients with schizophrenia who experience frequent auditory hallucinations fail to demonstrate an expected right ear advantage on the dichotic listening test. Absence of a right ear advantage is indicative of a functional deficit in the left peri-Sylvian region. The results also revealed that patients with ongoing auditory hallucinations were more impaired than patients with previous hallucinations, and that a higher score on the hallucination item in a standard symptom rating scale (BPRS) correlated negatively with number of correct reports for the right ear stimulus. Moreover, we have found that schizophrenia patients fail to shift attention to the left ear stimulus, when explicitly instructed to focus on the right or left ear stimulus only, thus showing a deficit in inhibition of attention and response-inhibition. The behavioral DL data are substantiated in two MR morphometry studies that revealed significant reductions in grey matter density in the left peri-Sylvian region in hallucinating patients, and patients with reduced left temporal lobe grey matter density. Hallucinating patients also failed to show a right ear advantage in the dichotic listening test. Ongoing fMRI studies are focused on the underlying synaptic and molecular mechanisms by investigating the effects of the glutamate antagonist drug memantine on auditory perception and speech lateralization, and examination of temporal cortex-specific gene expression in the left peri-Sylvian region.
Keywords: VBM; auditory hallucinations; dichotic listening; fMRI; hemisphere asymmetry; memantine; schizophrenia.
Figures




References
-
- American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM). Washington, D.C.
-
- Bartha R., Al-Semaan Y. M., Williamson P. C., Drost D. J., Malla A. K., Carr T. J., Densmore M., Canaran G., Neufeld W. R. J. (1999). A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biol. Psychiatry 45, 1403–141110.1016/S0006-3223(99)00007-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources

