HSV gene transfer in the treatment of chronic pain
- PMID: 18958369
- PMCID: PMC2586061
HSV gene transfer in the treatment of chronic pain
Abstract
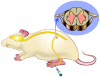
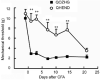
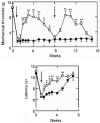
It has proven difficult to use systemic administration of small molecules to selectively modulate nociception. Over the past decade, we and others have developed non-replicating herpes simplex virus (HSV)-based vectors to treat chronic pain. Subcutaneous inoculation of an HSV vector effectively transduces sensory neurons in the dorsal root ganglion; release of transgene-coded inhibitory neurotransmitters or anti-inflammatory peptides reduces pain-related behaviors in rodent models of chronic inflammatory and neuropathic pain. A phase 1 trial of this therapy in patients is set to begin soon.
Figures

Similar articles
-
Development of HSV-mediated gene transfer for the treatment of chronic pain.Exp Neurol. 2003 Nov;184 Suppl 1:S25-9. doi: 10.1016/s0014-4886(03)00357-1. Exp Neurol. 2003. PMID: 14597322
-
HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain.Mol Pain. 2006 Feb 17;2:6. doi: 10.1186/1744-8069-2-6. Mol Pain. 2006. PMID: 16503976 Free PMC article.
-
Gene Transfer of Glutamic Acid Decarboxylase 67 by Herpes Simplex Virus Vectors Suppresses Neuropathic Pain Induced by Human Immunodeficiency Virus gp120 Combined with ddC in Rats.Anesth Analg. 2015 Jun;120(6):1394-404. doi: 10.1213/ANE.0000000000000729. Anesth Analg. 2015. PMID: 25851180
-
Exploiting the neurotherapeutic potential of peptides: targeted delivery using HSV vectors.Expert Opin Biol Ther. 2003 Dec;3(8):1233-9. doi: 10.1517/14712598.3.8.1233. Expert Opin Biol Ther. 2003. PMID: 14640949 Review.
-
A human trial of HSV-mediated gene transfer for the treatment of chronic pain.Gene Ther. 2009 Apr;16(4):455-60. doi: 10.1038/gt.2009.17. Epub 2009 Feb 26. Gene Ther. 2009. PMID: 19242524 Free PMC article. Review.
Cited by
-
Intrathecal administration of human bone marrow mesenchymal stem cells genetically modified with human proenkephalin gene decrease nociceptive pain in neuropathic rats.Mol Pain. 2017 Jan;13:1744806917701445. doi: 10.1177/1744806917701445. Mol Pain. 2017. PMID: 28326940 Free PMC article.
-
Development of viral vectors for gene therapy for chronic pain.Pain Res Treat. 2011;2011:968218. doi: 10.1155/2011/968218. Epub 2011 Apr 7. Pain Res Treat. 2011. PMID: 22110937 Free PMC article.
References
-
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. - PubMed
-
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. - PubMed
-
- Borsook D, Ploghaus A, Becerra L. Utilizing brain imaging for analgesic drug development. Curr Opin Investig Drugs. 2002;3(9):1342–1347. - PubMed
-
- Casey KL, Lorenz J, Minoshima S. Insights into the pathophysiology of neuropathic pain through functional brain imaging. Exp Neurol. 2003;184(Suppl 1):S80–88. - PubMed
-
- Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

