Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations
- PMID: 18958403
- PMCID: PMC2628956
- DOI: 10.1007/s11095-008-9752-7
Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations
Abstract
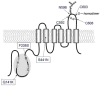
Purpose: Single nucleotide polymorphisms (SNPs) of the ATP-binding cassette (ABC) transporter ABCG2 gene have been suggested to be a significant factor in patients' responses to medication and/or the risk of diseases. We aimed to evaluate the impact of the major non-synonymous SNP Q141K on lysosomal and proteasomal degradations.
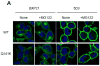
Methods: ABCG2 WT and the Q141K variant were expressed in Flp-In-293 cells by using the Flp recombinase system. Their expression levels and cellular localization was measured by immunoblotting and immunofluorescence microscopy, respectively.
Results: The protein level of the Q141K variant expressed in Flp-In-293 cells was about half that of ABCG2 WT, while their mRNA levels were equal. The protein expression level of the Q141K variant increased about two-fold when Flp-In-293 cells were treated with MG132. In contrast, the protein level of ABCG2 WT was little affected by the same treatment. After treatment with bafilomycin A1, the protein levels of ABCG2 WT and Q141K increased 5- and 2-fold in Flp-In-293 cells, respectively.
Conclusions: The results strongly suggest that the major non-synonymous SNP Q141K affects the stability of the ABCG2 protein in the endoplasmic reticulum and enhances its susceptibility to ubiquitin-mediated proteasomal degradation.
Figures








References
-
- Kalow E, Meyer U, Tyndale RF. Pharmacogenomics. Marcel Dekker; New York Besel: 2001.
-
- Ishikawa T, Tamura A, Saito H, Wakabayashi K, Nakagawa H. Pharmacogenomics of the human ABC transporter ABCG2: from functional evaluation to drug molecular design. Naturwissenschaften. 2005:1–13. - PubMed
-
- de Jong FA, de Jonge MJ, Verweij J, Mathijssen RH. Role of pharmacogenetics in irinotecan therapy. Cancer Lett. 2006;234:90–106. - PubMed
-
- Yanase K, Tsukahara S, Mitsuhashi J, Sugimoto Y. Functional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor development. Cancer Lett. 2006;234:73–80. - PubMed
-
- Backstrom G, Taipalensuu J, Melhus H, Brandstrom H, Svensson AC, Artursson P, Kindmark A. Genetic variation in the ATP-binding cassette transporter gene ABCG2 (BCRP) in a Swedish population. Eur J Pharm Sci. 2003;18:359–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

