Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells
- PMID: 18958626
- PMCID: PMC3764920
- DOI: 10.1007/s11481-008-9128-0
Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells
Abstract
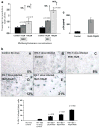
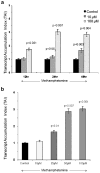
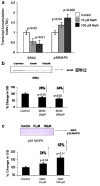
The US is currently experiencing an epidemic of methamphetamine (Meth) use as a recreational drug. Recent studies also show a high prevalence of HIV-1 infection among Meth users. We report that Meth enhances HIV-1 infectivity of dendritic cells as measured by multinuclear activation of a galactosidase indicator (MAGI) cell assay, p24 assay, and LTR-RU5 amplification. Meth induces increased HIV-1 infection in association with an increase in the HIV-1 coreceptors, CXCR4 and CCR5, and infection is mediated by downregulation of extracellular-regulated kinase (ERK2) and the upregulation of p38 mitogen-activated protein kinase (MAPK). A p38 inhibitor (SB203580) specifically reversed the Meth-induced upregulation of the CCR5 HIV-1 coreceptor. The dopamine D2 receptor antagonist RS +/- sulpiride significantly reversed the Meth-induced upregulation of CCR5, demonstrating that the Meth-induced effect is mediated via the D2 receptor. These studies report for the first time that Meth fosters HIV-1 infection, potentially via upregulating coreceptor gene expression. Further, Meth mediates its regulatory effects via dopamine receptors and via downregulating ERK2 with a reciprocal upregulation of p38 MAPK. Elucidation of the role of Meth in HIV-1 disease susceptibility and the mechanism through which Meth mediates its effects on HIV-1 infection may help to devise novel therapeutic strategies against HIV-1 infection in high-risk Meth-using HIV-1-infected subjects.
Figures


References
-
- Baselt RC. Disposition of toxic drugs and chemicals in man. 2. Vol. 38. Biomedical Publications; Davis: 1982. p. 113.
-
- Cameron P, Pope M, Granelli-Piperno A, Steinman RM. Dendritic cells and the replication of HIV-1. J Leukoc Biol. 1996;59:158–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

