Design of thiolate rich metal binding sites within a peptidic framework
- PMID: 18959366
- PMCID: PMC2650386
- DOI: 10.1021/ic8009817
Design of thiolate rich metal binding sites within a peptidic framework
Abstract
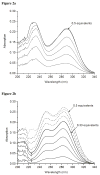
A de novo protein design strategy provides a powerful tool to elucidate how heavy metals interact with proteins.Cysteine derivatives of the TRI peptide family (Ac-G(LKALEEK)4G-NH2) have been shown to bind heavy metals in an unusual trigonal geometry. Our present objective was to design binding sites in R-helical scaffolds that are able to form higher coordination number complexes with Cd(II) and Hg(II). Herein, we evaluate the binding of Cd(II) and Hg(II) to double cysteine substituted TRI peptides lacking intervening leucines between sulfurs in the heptads. We compare a -Cysd-X-X-X-Cysa- binding motif found in TRIL12CL16C to the more common -Cysa-X-X-Cysd- sequence of native proteins found in TRIL9CL12C. Compared to TRI, these substitutions destabilize the helical aggregates,leading to mixtures of two- and three-stranded bundles. The three-stranded coiled coils are stabilized by the addition of metals. TRIL9CL12C forms distorted tetrahedral complexes with both Cd(II) and Hg(II), as supported by UV-vis,CD, 113Cd NMR, 199Hg NMR and 111mCd PAC spectroscopy. Additionally, these signatures are very similar to those found for heavy metal substituted rubredoxin. These results suggest that in terms of Hg(II) binding, TRIL9CL12Ccan be considered as a good mimic of the metallochaperone HAH1, that has previously been shown to form protein dimers. TRIL12CL16C has limited ability to generate homoleptic tetrahedral complexes (Cd(SR)42-). These type of complexes were identified only for Hg(II). However, the spectroscopic signatures suggest a different geometry around the metal ion, demonstrating that effective metal sequestration into the hydrophobic interior of the bundle requires more than simply adding two sulfur residues in adjacent layers of the peptide core. Thus, proper design of metal binding sites must also consider the orientation of cysteine sidechains in a vs d positions of the heptads.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

