The effect of universal influenza immunization on mortality and health care use
- PMID: 18959473
- PMCID: PMC2573914
- DOI: 10.1371/journal.pmed.0050211
The effect of universal influenza immunization on mortality and health care use
Abstract
Background: In 2000, Ontario, Canada, initiated a universal influenza immunization program (UIIP) to provide free influenza vaccines for the entire population aged 6 mo or older. Influenza immunization increased more rapidly in younger age groups in Ontario compared to other Canadian provinces, which all maintained targeted immunization programs. We evaluated the effect of Ontario's UIIP on influenza-associated mortality, hospitalizations, emergency department (ED) use, and visits to doctors' offices.
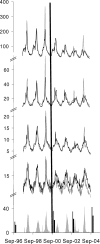
Methods and findings: Mortality and hospitalization data from 1997 to 2004 for all ten Canadian provinces were obtained from national datasets. Physician billing claims for visits to EDs and doctors' offices were obtained from provincial administrative datasets for four provinces with comprehensive data. Since outcomes coded as influenza are known to underestimate the true burden of influenza, we studied more broadly defined conditions. Hospitalizations, ED use, doctors' office visits for pneumonia and influenza, and all-cause mortality from 1997 to 2004 were modelled using Poisson regression, controlling for age, sex, province, influenza surveillance data, and temporal trends, and used to estimate the expected baseline outcome rates in the absence of influenza activity. The primary outcome was then defined as influenza-associated events, or the difference between the observed events and the expected baseline events. Changes in influenza-associated outcome rates before and after UIIP introduction in Ontario were compared to the corresponding changes in other provinces. After UIIP introduction, influenza-associated mortality decreased more in Ontario (relative rate [RR] = 0.26) than in other provinces (RR = 0.43) (ratio of RRs = 0.61, p = 0.002). Similar differences between Ontario and other provinces were observed for influenza-associated hospitalizations (RR = 0.25 versus 0.44, ratio of RRs = 0.58, p < 0.001), ED use (RR = 0.31 versus 0.69, ratio of RRs = 0.45, p < 0.001), and doctors' office visits (RR = 0.21 versus 0.52, ratio of RRs = 0.41, p < 0.001). Sensitivity analyses were carried out to assess consistency, specificity, and the presence of a dose-response relationship. Limitations of this study include the ecological study design, the nonspecific outcomes, difficulty in modeling baseline events, data quality and availability, and the inability to control for potentially important confounders.
Conclusions: Compared to targeted programs in other provinces, introduction of universal vaccination in Ontario in 2000 was associated with relative reductions in influenza-associated mortality and health care use. The results of this large-scale natural experiment suggest that universal vaccination may be an effective public health measure for reducing the annual burden of influenza.
Conflict of interest statement
Figures

Comment in
-
Health benefits of universal influenza vaccination strategy.PLoS Med. 2008 Oct 28;5(10):e216. doi: 10.1371/journal.pmed.0050216. PLoS Med. 2008. PMID: 18959475 Free PMC article.
References
-
- Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–1775. - PubMed
-
- Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. - PubMed
-
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. - PubMed
-
- Jefferson TO, Rivetti D, Di PC, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007. CD001269. - PubMed
-
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008. CD004879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

