The desensitization gating of the MthK K+ channel is governed by its cytoplasmic amino terminus
- PMID: 18959476
- PMCID: PMC2573919
- DOI: 10.1371/journal.pbio.0060223
The desensitization gating of the MthK K+ channel is governed by its cytoplasmic amino terminus
Abstract
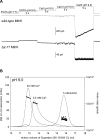
The RCK-containing MthK channel undergoes two inactivation processes: activation-coupled desensitization and acid-induced inactivation. The acid inactivation is mediated by the C-terminal RCK domain assembly. Here, we report that the desensitization gating is governed by a desensitization domain (DD) of the cytoplasmic N-terminal 17 residues. Deletion of DD completely removes the desensitization, and the process can be fully restored by a synthetic DD peptide added in trans. Mutagenesis analyses reveal a sequence-specific determinant for desensitization within the initial hydrophobic segment of DD. Proton nuclear magnetic resonance ((1)H NMR) spectroscopy analyses with synthetic peptides and isolated RCK show interactions between the two terminal domains. Additionally, we show that deletion of DD does not affect the acid-induced inactivation, indicating that the two inactivation processes are mutually independent. Our results demonstrate that the short N-terminal DD of MthK functions as a complete moveable module responsible for the desensitization. Its interaction with the C-terminal RCK domain may play a role in the gating process.
Conflict of interest statement
Figures






References
-
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. - PubMed
-
- Hille B. Ion channels of excitable membranes. Sunderland (Massachusetts): Sinauer Associates; 2001. 814
-
- Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, et al. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. - PubMed
-
- Roosild TP, Le KT, Choe S. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends Biochem Sci. 2004;29:39–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

