An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA
- PMID: 18959477
- PMCID: PMC2567003
- DOI: 10.1371/journal.pbio.0060249
An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA
Abstract
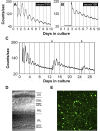
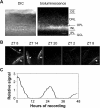
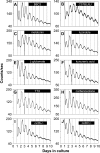
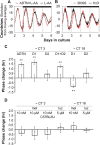
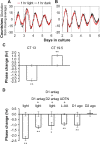
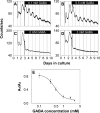
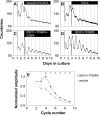
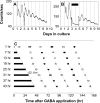
The influence of the mammalian retinal circadian clock on retinal physiology and function is widely recognized, yet the cellular elements and neural regulation of retinal circadian pacemaking remain unclear due to the challenge of long-term culture of adult mammalian retina and the lack of an ideal experimental measure of the retinal circadian clock. In the current study, we developed a protocol for long-term culture of intact mouse retinas, which allows retinal circadian rhythms to be monitored in real time as luminescence rhythms from a PERIOD2::LUCIFERASE (PER2::LUC) clock gene reporter. With this in vitro assay, we studied the characteristics and location within the retina of circadian PER2::LUC rhythms, the influence of major retinal neurotransmitters, and the resetting of the retinal circadian clock by light. Retinal PER2::LUC rhythms were routinely measured from whole-mount retinal explants for 10 d and for up to 30 d. Imaging of vertical retinal slices demonstrated that the rhythmic luminescence signals were concentrated in the inner nuclear layer. Interruption of cell communication via the major neurotransmitter systems of photoreceptors and ganglion cells (melatonin and glutamate) and the inner nuclear layer (dopamine, acetylcholine, GABA, glycine, and glutamate) did not disrupt generation of retinal circadian PER2::LUC rhythms, nor did interruption of intercellular communication through sodium-dependent action potentials or connexin 36 (cx36)-containing gap junctions, indicating that PER2::LUC rhythms generation in the inner nuclear layer is likely cell autonomous. However, dopamine, acting through D1 receptors, and GABA, acting through membrane hyperpolarization and casein kinase, set the phase and amplitude of retinal PER2::LUC rhythms, respectively. Light pulses reset the phase of the in vitro retinal oscillator and dopamine D1 receptor antagonists attenuated these phase shifts. Thus, dopamine and GABA act at the molecular level of PER proteins to play key roles in the organization of the retinal circadian clock.
Conflict of interest statement
Figures


References
-
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. - PubMed
-
- Grace MS, Wang LM, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735:93–100. - PubMed
-
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. - PubMed
-
- Grace MS, Chiba A, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999;16:909–918. - PubMed
-
- Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

