Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system
- PMID: 18959479
- PMCID: PMC2573929
- DOI: 10.1371/journal.pbio.0060255
Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system
Abstract
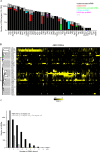
RNA-binding proteins (RBPs) have roles in the regulation of many post-transcriptional steps in gene expression, but relatively few RBPs have been systematically studied. We searched for the RNA targets of 40 proteins in the yeast Saccharomyces cerevisiae: a selective sample of the approximately 600 annotated and predicted RBPs, as well as several proteins not annotated as RBPs. At least 33 of these 40 proteins, including three of the four proteins that were not previously known or predicted to be RBPs, were reproducibly associated with specific sets of a few to several hundred RNAs. Remarkably, many of the RBPs we studied bound mRNAs whose protein products share identifiable functional or cytotopic features. We identified specific sequences or predicted structures significantly enriched in target mRNAs of 16 RBPs. These potential RNA-recognition elements were diverse in sequence, structure, and location: some were found predominantly in 3'-untranslated regions, others in 5'-untranslated regions, some in coding sequences, and many in two or more of these features. Although this study only examined a small fraction of the universe of yeast RBPs, 70% of the mRNA transcriptome had significant associations with at least one of these RBPs, and on average, each distinct yeast mRNA interacted with three of the RBPs, suggesting the potential for a rich, multidimensional network of regulation. These results strongly suggest that combinatorial binding of RBPs to specific recognition elements in mRNAs is a pervasive mechanism for multi-dimensional regulation of their post-transcriptional fate.
Conflict of interest statement
Figures





References
-
- Hieronymus H, Silver PA. A systems view of mRNP biology. Genes Dev. 2004;18:2845–2860. - PubMed
-
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. - PubMed
-
- Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. - PubMed
-
- Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell. 2002;9:1161–1167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

