Profound context-dependent plasticity of mitral cell responses in olfactory bulb
- PMID: 18959481
- PMCID: PMC2573932
- DOI: 10.1371/journal.pbio.0060258
Profound context-dependent plasticity of mitral cell responses in olfactory bulb
Abstract
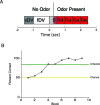
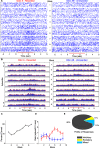
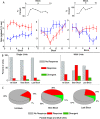
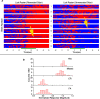
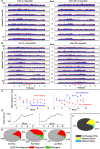
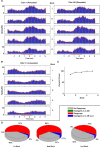
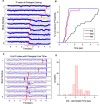
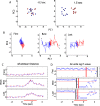
On the basis of its primary circuit it has been postulated that the olfactory bulb (OB) is analogous to the retina in mammals. In retina, repeated exposure to the same visual stimulus results in a neural representation that remains relatively stable over time, even as the meaning of that stimulus to the animal changes. Stability of stimulus representation at early stages of processing allows for unbiased interpretation of incoming stimuli by higher order cortical centers. The alternative is that early stimulus representation is shaped by previously derived meaning, which could allow more efficient sampling of odor space providing a simplified yet biased interpretation of incoming stimuli. This study helps place the olfactory system on this continuum of subjective versus objective early sensory representation. Here we show that odor responses of the output cells of the OB, mitral cells, change transiently during a go-no-go odor discrimination task. The response changes occur in a manner that increases the ability of the circuit to convey information necessary to discriminate among closely related odors. Remarkably, a switch between which of the two odors is rewarded causes mitral cells to switch the polarity of their divergent responses. Taken together these results redefine the function of the OB as a transiently modifiable (active) filter, shaping early odor representations in behaviorally meaningful ways.
Conflict of interest statement
Figures

References
-
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. - PubMed
-
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. - PubMed
-
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. - PubMed
-
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The synaptic organization of the brain. New York: Oxford University Press; 2004. pp. 159–204.
-
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

