Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation
- PMID: 18962898
- PMCID: PMC2760063
- DOI: 10.1016/j.molimm.2008.09.018
Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation
Abstract
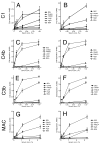
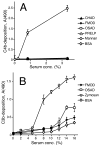
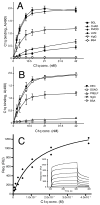
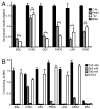
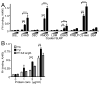
The extracellular matrix consists of structural macromolecules and other proteins with regulatory functions. An important family of the latter class of molecules found in most tissues is the small leucine-rich repeat proteins (SLRPs). We have previously shown that the SLRP fibromodulin binds directly to C1q and activates the classical pathway of complement. In the present study we further examine the interactions between SLRPs and complement. Osteoadherin, like fibromodulin, binds C1q and activates the classical pathway strongly while moderate activation is seen in the terminal pathway. This can be explained by the interaction of fibromodulin and osteoadherin with factor H, a major soluble inhibitor of complement. Also, chondroadherin was found to bind C1q and activate complement, albeit to a lesser extent. Chondroadherin also binds factor H. We confirm published data showing that biglycan and decorin bind C1q but do not activate complement. In this study a similar pattern is seen for lumican although its affinity for C1q is lower than for biglycan and decorin. Furthermore, using electron microscopy and radiolabeled SLRPs, we demonstrate two different classes of SLRP binding sites on C1q, to head and stalk respectively, where only binding to the head appears to be activating. We propose a role for SLRPs in the regulation of complement activation in diseases involving the extracellular matrix, particularly those characterized by chronic inflammation such as rheumatoid arthritis, atherosclerosis, osteoarthritis and chronic obstructive lung disease.
Figures


References
-
- Antonsson P, Heinegård D, Oldberg A. Posttranslational modifications of fibromodulin. J Biol Chem. 1991;266:16859–61. - PubMed
-
- Aronen M, Lehto T, Meri S. Regulation of alternative pathway complement activation by an interaction of C-reactive protein with factor H. Immunology and Infectious Diseases. 1992;3:83–87.
-
- Baschong W, Lucocq JM, Roth J. “Thiocyanate gold”: small (2–3 nm) colloidal gold for affinity cytochemical labeling in electron microscopy. Histochemistry. 1985;83:409–11. - PubMed
-
- Blom AM, Kask L, Dahlbäck B. CCP1–4 of the C4b-binding protein α-chain are required for Factor I mediated cleavage of C3b. Mol Immunol. 2003a;39:547–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

