Gene-specific control of the TLR-induced inflammatory response
- PMID: 18964303
- PMCID: PMC3252731
- DOI: 10.1016/j.clim.2008.08.015
Gene-specific control of the TLR-induced inflammatory response
Abstract
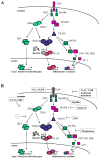
Toll-like receptors (TLRs) induce a complex inflammatory response that functions to alert the body to infection, neutralize pathogens, and repair damaged tissues. An excessive or persistent inflammatory response can be fatal, so multiple regulatory mechanisms have evolved to control the extent and duration of inflammation. Our current understanding of the control of inflammation is based on negative regulation of TLR signaling. However, TLR-induced genes have diverse functions, and control of signaling pathways does not allow for groups of genes with distinct functions to be differentially regulated. Recent evidence suggests that many inflammatory genes are instead regulated by epigenetic modifications to individual promoters. This level of control allows a single gene to be expressed or silenced according to its function, irrespective of other genes induced by the same receptor, and therefore is "gene-specific." Gene-specific control of the TLR-induced inflammatory response is an emerging paradigm in the study of inflammation, and may provide the basis for selective modulation of the inflammatory response.
Figures


References
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. - PubMed
-
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. - PubMed
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. - PubMed
-
- Yeaman MR, Yount NY. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5:727–40. - PubMed
-
- Berczi I. Neurohormonal host defense in endotoxin shock. Ann N Y Acad Sci. 1998;840:787–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

