Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques
- PMID: 18971271
- PMCID: PMC2612365
- DOI: 10.1128/JVI.01672-08
Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques
Abstract
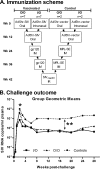
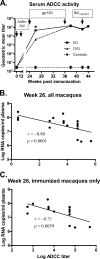
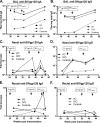
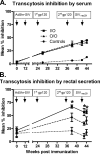
Cell-mediated immunity and neutralizing antibodies contribute to control of human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) infection, but the role of nonneutralizing antibodies is not defined. Previously, we reported that sequential oral/oral or intranasal/oral (I/O) priming with replication-competent adenovirus type 5 host range mutant (Ad5hr)-SIV recombinants, followed by intramuscular envelope protein boosting, elicited systemic and mucosal cellular immunity and exhibited equivalent, significant reductions of chronic viremia after rectal SIV(mac251) challenge. However, I/O priming gave significantly better control of acute viremia. Here, systemic and mucosal humoral immunity were investigated for potential correlates with the acute challenge outcome. Strong serum binding but nonneutralizing antibody responses against SIV(mac251) were induced in both groups. Antibody responses appeared earlier and overall were higher in the I/O group. Reduced acute viremia was significantly correlated with higher serum binding titer, stronger antibody-dependent cellular cytotoxicity activity, and peak prechallenge and 2-week-postchallenge antibody-dependent cell-mediated viral inhibition (ADCVI). The I/O group consistently displayed greater anti-envelope immunoglobulin A (IgA) antibody responses in bronchoalveolar lavage and a stronger rectal anti-envelope IgA anamnestic response 2 weeks postchallenge. Pre- and postchallenge rectal secretions inhibited SIV transcytosis across epithelial cells. The inhibition was significantly higher in the I/O group, although a significant correlation with reduced acute viremia was not reached. Overall, the replicating Ad5hr-SIV priming/envelope boosting approach elicited strong systemic and mucosal antibodies with multiple functional activities. The pattern of elevated immune responses in the I/O group is consistent with its better control of acute viremia mediated, at least in part, by ADCVI activity and transcytosis inhibition.
Figures


References
-
- Ahmad, A., and J. Menezes. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10258-266. - PubMed
-
- Ahmad, R., S. T. Sindhu, E. Toma, R. Morisset, J. Vincelette, J. Menezes, and A. Ahmad. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21227-233. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine 6200-206. - PubMed
-
- Banks, N. D., N. Kinsey, J. Clements, and J. E. Hildreth. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum. Retrovir. 181197-1205. - PubMed
-
- Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 342-47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

