Bromodomain protein Brd4 regulates human immunodeficiency virus transcription through phosphorylation of CDK9 at threonine 29
- PMID: 18971272
- PMCID: PMC2612389
- DOI: 10.1128/JVI.01316-08
Bromodomain protein Brd4 regulates human immunodeficiency virus transcription through phosphorylation of CDK9 at threonine 29
Abstract
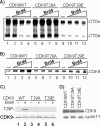
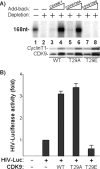
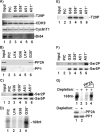
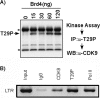
Positive transcription elongation factor b (P-TEFb), composed of cyclin-dependent kinase 9 (CDK9) and cyclin T, is a global transcription factor for eukaryotic gene expression, as well as a key factor for human immunodeficiency virus (HIV) transcription elongation. P-TEFb phosphorylates the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II (RNAP II), facilitating the transition from nonprocessive to processive transcription elongation. Recently, the bromodomain protein Brd4 has been shown to interact with the low-molecular-weight, active P-TEFb complex and recruit P-TEFb to the HIV type 1 long terminal repeat (LTR) promoter. However, the subsequent events through which Brd4 regulates CDK9 kinase activity and RNAP II-dependent transcription are not clearly understood. Here we provide evidence that Brd4 regulates P-TEFb kinase activity by inducing a negative pathway. Moreover, by analyzing stepwise initiation and elongation complexes, we demonstrate that P-TEFb activity is regulated in the transcription complex. Brd4 induces phosphorylation of CDK9 at threonine 29 (T29) in the HIV transcription initiation complex, inhibiting CDK9 kinase activity. P-TEFb inhibition is transient, as Brd4 is released from the transcription complex between positions +14 and +36. Removal of the phosphate group at T29 by an incoming phosphatase released P-TEFb activity, resulting in increased RNAP II CTD phosphorylation and transcription. Finally, we present chromatin immunoprecipitation studies showing that CDK9 with phosphorylated T29 is associated with the HIV promoter region in the integrated and transcriptionally silent HIV genome.
Figures


References
-
- Buratowski, S. 2003. The CTD code. Nat. Struct. Biol. 10679-680. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

