Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1
- PMID: 18971278
- PMCID: PMC2612316
- DOI: 10.1128/JVI.01032-08
Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1
Abstract
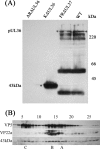
Studies with herpes simplex virus type 1 (HSV-1) have shown that secondary envelopment and virus release are blocked in mutants deleted for the tegument protein gene UL36 or UL37, leading to the accumulation of DNA-containing capsids in the cytoplasm of infected cells. The failure to assemble infectious virions has meant that the roles of these genes in the initial stages of infection could not be investigated. To circumvent this, cells infected at a low multiplicity were fused to form syncytia, thereby allowing capsids released from infected nuclei access to uninfected nuclei without having to cross a plasma membrane. Visualization of virus DNA replication showed that a UL37-minus mutant was capable of transmitting infection to all the nuclei within a syncytium as efficiently as the wild-type HSV-1 strain 17(+) did, whereas infection by UL36-minus mutants failed to spread. Thus, these inner tegument proteins have differing functions, with pUL36 being essential during both the assembly and uptake stages of infection, while pUL37 is needed for the formation of virions but is not required during the initial stages of infection. Analysis of noninfectious enveloped particles (L-particles) further showed that pUL36 and pUL37 are dependent on each other for incorporation into tegument.
Figures






Similar articles
-
Conserved Tryptophan Motifs in the Large Tegument Protein pUL36 Are Required for Efficient Secondary Envelopment of Herpes Simplex Virus Capsids.J Virol. 2016 May 12;90(11):5368-5383. doi: 10.1128/JVI.03167-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009950 Free PMC article.
-
The interaction of the HSV-1 tegument proteins pUL36 and pUL37 is essential for secondary envelopment during viral egress.Virology. 2014 Apr;454-455:67-77. doi: 10.1016/j.virol.2014.02.003. Epub 2014 Feb 22. Virology. 2014. PMID: 24725933
-
Cytosolic herpes simplex virus capsids not only require binding inner tegument protein pUL36 but also pUL37 for active transport prior to secondary envelopment.Cell Microbiol. 2013 Feb;15(2):248-69. doi: 10.1111/cmi.12075. Epub 2012 Dec 20. Cell Microbiol. 2013. PMID: 23186167
-
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses.Viruses. 2015 Sep 18;7(9):5084-114. doi: 10.3390/v7092861. Viruses. 2015. PMID: 26393641 Free PMC article. Review.
-
Functional roles of the tegument proteins of herpes simplex virus type 1.Virus Res. 2009 Nov;145(2):173-86. doi: 10.1016/j.virusres.2009.07.007. Epub 2009 Jul 15. Virus Res. 2009. PMID: 19615419 Review.
Cited by
-
The major determinant for addition of tegument protein pUL48 (VP16) to capsids in herpes simplex virus type 1 is the presence of the major tegument protein pUL36 (VP1/2).J Virol. 2010 Feb;84(3):1397-405. doi: 10.1128/JVI.01721-09. Epub 2009 Nov 18. J Virol. 2010. PMID: 19923173 Free PMC article.
-
Functional Domains of the Herpes Simplex Virus Type 1 Tegument Protein pUL37: The Amino Terminus is Dispensable for Virus Replication in Tissue Culture.Viruses. 2019 Sep 14;11(9):853. doi: 10.3390/v11090853. Viruses. 2019. PMID: 31540043 Free PMC article.
-
Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis.J Virol. 2010 Feb;84(3):1504-12. doi: 10.1128/JVI.02029-09. Epub 2009 Nov 18. J Virol. 2010. PMID: 19923187 Free PMC article.
-
Mutational analysis of the herpes simplex virus type 1 UL25 DNA packaging protein reveals regions that are important after the viral DNA has been packaged.J Virol. 2010 May;84(9):4252-63. doi: 10.1128/JVI.02442-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181717 Free PMC article.
-
Role of Nectin-1 and Herpesvirus Entry Mediator as Cellular Receptors for Herpes Simplex Virus 1 on Primary Murine Dermal Fibroblasts.J Virol. 2015 Sep;89(18):9407-16. doi: 10.1128/JVI.01415-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136572 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

