Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells
- PMID: 18971284
- PMCID: PMC2612309
- DOI: 10.1128/JVI.01652-08
Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells
Abstract
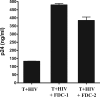
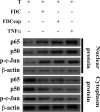
HIV replication occurs throughout the natural course of infection in secondary lymphoid tissues and in particular within the germinal centers (GCs), where follicular dendritic cells (FDCs) are adjacent to CD4(+) T cells. Because FDCs provide signaling that increases lymphocyte activation, we postulated that FDCs could increase human immunodeficiency virus (HIV) replication. We cultured HIV-infected CD4(+) T cells alone or with FDCs and measured subsequent virus expression using HIV-p24 production and reverse transcription-PCR analyses. When cultured with FDCs, infected CD4(+) T cells produced almost fourfold more HIV than when cultured alone, and the rate of virus transcription was doubled. Both FDCs and their supernatant increased HIV transcription and resulted in nuclear translocation of NF-kappaB and phosphorylated c-Jun in infected cells. FDCs produced soluble tumor necrosis factor alpha (TNF-alpha) ex vivo, and the addition of a blocking soluble TNF receptor ablated FDC-mediated HIV transcription. Furthermore, TNF-alpha was found highly expressed within GCs, and ex vivo GC CD4(+) T cells supported greater levels of HIV-1 replication than other CD4(+) T cells. These data indicated that FDCs increase HIV transcription and production by a soluble TNF-alpha-mediated mechanism. This FDC-mediated effect may account, at least in part, for the presence of persistent HIV replication in GCs. Therefore, in addition to providing an important reservoir of infectious virus, FDCs increase HIV production, contributing to a tissue microenvironment that is highly conducive to HIV transmission and expression.
Figures





References
-
- Armstrong, J. A. 1991. Ultrastructure and significance of the lymphoid tissue lesions in HIV infection, p. 69-82. In P. Racz, C. D. Dijkstra, and J. C. Gluckman (ed.), Accessory cells in HIV and other retroviral infections. Karger, Basel, Switzerland.
-
- Biberfeld, P., A. Porwit, G. Biberfield, M. Harper, A. Bodner, and R. Gallo. 1988. Lymphadenopathy in HIV (HTLV-III LAV) infected subjects: the role of virus and follicular dendritic cells. Cancer Detect. Prev. 12217-224. - PubMed
-
- Burton, G. F., B. F. Keele, J. D. Estes, T. C. Thacker, and S. Gartner. 2002. Follicular dendritic cell contributions to HIV pathogenesis. Semin. Immunol. 14275-284. - PubMed
-
- Burton, G. F., A. Masuda, S. L. Heath, B. A. Smith, J. G. Tew, and A. K. Szakal. 1997. Follicular dendritic cells (FDC) in retroviral infection: host/pathogen perspectives. Immunol. Rev. 156185-197. - PubMed
-
- Butch, A. W., G. H. Chung, J. W. Hoffmann, and M. H. Nahm. 1993. Cytokine expression by germinal center cells. J. Immunol. 15039-47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

