Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells
- PMID: 18971378
- PMCID: PMC2613126
- DOI: 10.1091/mbc.e08-04-0372
Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells
Abstract
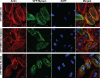
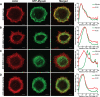
To better understand the mechanism controlling nonmuscle myosin II (NM-II) assembly in mammalian cells, mutant NM-IIA constructs were created to allow tests in live cells of two widely studied models for filament assembly control. A GFP-NM-IIA construct lacking the RLC binding domain (DeltaIQ2) destabilizes the 10S sequestered monomer state and results in a severe defect in recycling monomers during spreading, and from the posterior to the leading edge during polarized migration. A GFP-NM-IIA construct lacking the nonhelical tailpiece (Deltatailpiece) is competent for leading edge assembly, but overassembles, suggesting defects in disassembly from lamellae subsequent to initial recruitment. The Deltatailpiece phenotype was recapitulated by a GFP-NM-IIA construct carrying a mutation in a mapped tailpiece phosphorylation site (S1943A), validating the importance of the tailpiece and tailpiece phosphorylation in normal lamellar myosin II assembly control. These results demonstrate that both the 6S/10S conformational change and the tailpiece contribute to the localization and assembly of myosin II in mammalian cells. This work furthermore offers cellular insights that help explain platelet and leukocyte defects associated with R1933-stop alleles of patients afflicted with human MYH9-related disorder.
Figures







References
-
- Adelstein R. S., Pato M. D., Conti M. A. The role of phosphorylation in regulating contractile proteins. Adv. Cyclic. Nucleotide Res. 1981;14:361–373. - PubMed
-
- Bao J., Jana S. S., Adelstein R. S. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 2005;280:19594–19599. - PubMed
-
- Bray D., White J. G. Cortical flow in animal cells. Science. 1988;239:883–888. - PubMed
-
- Burgess S. A., Yu S., Walker M. L., Hawkins R. J., Chalovich J. M., Knight P. J. Structures of smooth muscle myosin and heavy meromyosin in the folded, shutdown state. J. Mol. Biol. 2007;372:1165–1178. - PubMed
-
- Conti M. A., Adelstein R. S. Nonmuscle myosin II moves in new directions. J. Cell Sci. 2008;121:11–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

