Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients
- PMID: 18971421
- PMCID: PMC2656271
- DOI: 10.1182/blood-2008-05-157222
Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients
Abstract
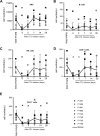
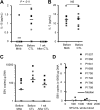
Treatment of Epstein-Barr virus (EBV)-positive nasopharyngeal carcinoma (NPC) with EBV-specific cytotoxic T cells (EBV-specific CTL) has been promising, producing clinical responses. However, infused EBV-specific CTL did not expand in vivo, likely limiting their antitumor activity. Lymphodepleting patients with chemotherapy before T-cell transfer enhances in vivo T-cell expansion, but results in nonspecific destruction of the resident immune system and can have significant toxicity. To evaluate if monoclonal antibodies (mAbs) can produce a more selective lymphodepletion, we conducted a clinical study in which NPC patients received a pair of lymphodepleting mAbs targeted to the CD45 antigen (CD45 mAbs) before EBV-specific CTL infusion. Eight patients with recurrent NPC received CD45 mAbs followed by escalating doses of autologous EBV-specific CTL. Infusion of CD45 mAbs resulted in transient lymphopenia in all patients and an increase in interleukin-15 (IL-15) levels in 6 out 8 patients. All patients had an increase in their peripheral blood frequency of EBV-specific T cells after CTL infusion. Three patients with a persistent increase had clinical benefits including 1 complete response (> 24 months) and 2 with stable disease (for 12 and 15 months). Lymphodepleting mAbs prior CTL transfer may represent an alternative to chemotherapy to enhance expansion of infused CTL. This study is registered at (http://www.clinialtrials.gov) as NCT00608257.
Figures



References
-
- Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–1015. - PubMed
-
- Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. - PubMed
-
- Mould RF, Tai TH. Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. Br J Radiol. 2002;75:307–339. - PubMed
-
- Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4:13–21. - PubMed
-
- Wang CC, Chen ML, Hsu KH, et al. Second malignant tumors in patients with nasopharyngeal carcinoma and their association with Epstein-Barr virus. Int J Cancer. 2000;87:228–231. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

