Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain
- PMID: 18971468
- PMCID: PMC6671487
- DOI: 10.1523/JNEUROSCI.2308-08.2008
Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain
Abstract
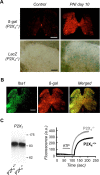
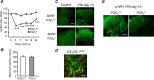
ATP is a known mediator of inflammatory and neuropathic pain. However, the mechanisms by which specific purinergic receptors contribute to chronic pain states are still poorly characterized. Here, we demonstrate that in response to peripheral nerve injury, P2X(4) receptors (P2X(4)R) are expressed de novo by activated microglia in the spinal cord. Using in vitro and in vivo models, we provide direct evidence that P2X(4)R stimulation leads to the release of BDNF from activated microglia and, most likely phosphorylation of the NR1 subunit of NMDA receptors in dorsal horn neurons of the spinal cord. Consistent with these findings, P2X4-deficient mice lack mechanical hyperalgesia induced by peripheral nerve injury and display impaired BDNF signaling in the spinal cord. Furthermore, ATP stimulation is unable to stimulate BDNF release from P2X(4)-deficient mice microglia in primary cultures. These results indicate that P2X(4)R contribute to chronic pain through a central inflammatory pathway. P2X(4)R might thus represent a potential therapeutic target to limit microglia-mediated inflammatory responses associated with brain injury and neurodegenerative disorders.
Figures


References
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Buell G, Michel AD, Lewis C, Collo G, Humphrey PP, Surprenant A. P2X1 receptor activation in HL60 cells. Blood. 1996;87:2659–2664. - PubMed
-
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. - PubMed
-
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. - PubMed
-
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
