TorsinA protein degradation and autophagy in DYT1 dystonia
- PMID: 18971629
- PMCID: PMC2606915
- DOI: 10.4161/auto.5.1.7173
TorsinA protein degradation and autophagy in DYT1 dystonia
Abstract
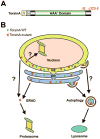
Early-onset generalized dystonia (DYT1) is a debilitating neurological disorder characterized by involuntary movements and sustained muscle spasms. DYT1 dystonia has been associated with two mutations in torsinA that result in the deletion of a single glutamate residue (torsinA DeltaE) and six amino-acid residues (torsinA Delta323-8). We recently revealed that torsinA, a peripheral membrane protein, which resides predominantly in the lumen of the endoplasmic reticulum (ER) and nuclear envelope (NE), is a long-lived protein whose turnover is mediated by basal autophagy. Dystonia-associated torsinA DeltaE and torsinA Delta323-8 mutant proteins show enhanced retention in the NE and accelerated degradation by both the proteasome and autophagy. Our results raise the possibility that the monomeric form of torsinA mutant proteins is cleared by proteasome-mediated ER-associated degradation (ERAD), whereas the oligomeric and aggregated forms of torsinA mutant proteins are cleared by ER stress-induced autophagy. Our findings provide new insights into the pathogenic mechanism of torsinA DeltaE and torsinA Delta323-8 mutations in dystonia and emphasize the need for a mechanistic understanding of the role of autophagy in protein quality control in the ER and NE compartments.
Figures
References
-
- Bressman SB, Fahn S, Ozelius LJ, Kramer PL, Risch NJ. The DYT1 mutation and nonfamilial primary torsion dystonia. Arch Neurol. 2001;58:681–2. - PubMed
-
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–8. - PubMed
-
- Leung JC, Klein C, Friedman J, Vieregge P, Jacobs H, Doheny D, Kamm C, DeLeon D, Pramstaller PP, Penney JB, Eisengart M, Jankovic J, Gasser T, Bressman SB, Corey DP, Kramer P, Brin MF, Ozelius LJ, Breakefield XO. Novel mutation in the TOR1A (DYT1) gene in atypical early onset dystonia and polymorphisms in dystonia and early onset parkinsonism. Neurogenetics. 2001;3:133–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
