A fast, robust and tunable synthetic gene oscillator
- PMID: 18971928
- PMCID: PMC6791529
- DOI: 10.1038/nature07389
A fast, robust and tunable synthetic gene oscillator
Abstract
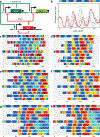
One defining goal of synthetic biology is the development of engineering-based approaches that enable the construction of gene-regulatory networks according to 'design specifications' generated from computational modelling. This approach provides a systematic framework for exploring how a given regulatory network generates a particular phenotypic behaviour. Several fundamental gene circuits have been developed using this approach, including toggle switches and oscillators, and these have been applied in new contexts such as triggered biofilm development and cellular population control. Here we describe an engineered genetic oscillator in Escherichia coli that is fast, robust and persistent, with tunable oscillatory periods as fast as 13 min. The oscillator was designed using a previously modelled network architecture comprising linked positive and negative feedback loops. Using a microfluidic platform tailored for single-cell microscopy, we precisely control environmental conditions and monitor oscillations in individual cells through multiple cycles. Experiments reveal remarkable robustness and persistence of oscillations in the designed circuit; almost every cell exhibited large-amplitude fluorescence oscillations throughout observation runs. The oscillatory period can be tuned by altering inducer levels, temperature and the media source. Computational modelling demonstrates that the key design principle for constructing a robust oscillator is a time delay in the negative feedback loop, which can mechanistically arise from the cascade of cellular processes involved in forming a functional transcription factor. The positive feedback loop increases the robustness of the oscillations and allows for greater tunability. Examination of our refined model suggested the existence of a simplified oscillator design without positive feedback, and we construct an oscillator strain confirming this computational prediction.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

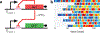

References
-
- Hasty J, Dolnik M, Rottschäfer V & Collins JJ Synthetic gene network for entraining and amplifying cellular oscillations. Phys Rev Lett 88, 148101 (2002). - PubMed
-
- Hasty J, McMillen D & Collins JJ Engineered gene circuits. Nature 420, 224–230 (2002). - PubMed
-
- Tyson J, Chen K & Novak B Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Current Opinion in Cell Biology 15, 221–231 (2003). - PubMed
-
- Sprinzak D & Elowitz MB Reconstruction of genetic circuits. Nature 438, 443–448 (2005). - PubMed
-
- Endy D Foundations for engineering biology. Nature 438, 449–453 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

