The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm
- PMID: 18971929
- PMCID: PMC2687078
- DOI: 10.1038/nature07526
The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm
Abstract
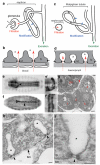
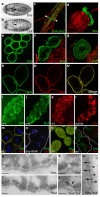
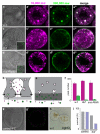
The nephron is the basic structural and functional unit of the vertebrate kidney. It is composed of a glomerulus, the site of ultrafiltration, and a renal tubule, along which the filtrate is modified. Although widely regarded as a vertebrate adaptation, 'nephron-like' features can be found in the excretory systems of many invertebrates, raising the possibility that components of the vertebrate excretory system were inherited from their invertebrate ancestors. Here we show that the insect nephrocyte has remarkable anatomical, molecular and functional similarity to the glomerular podocyte, a cell in the vertebrate kidney that forms the main size-selective barrier as blood is ultrafiltered to make urine. In particular, both cell types possess a specialized filtration diaphragm, known as the slit diaphragm in podocytes or the nephrocyte diaphragm in nephrocytes. We find that fly (Drosophila melanogaster) orthologues of the major constituents of the slit diaphragm, including nephrin, NEPH1 (also known as KIRREL), CD2AP, ZO-1 (TJP1) and podocin, are expressed in the nephrocyte and form a complex of interacting proteins that closely mirrors the vertebrate slit diaphragm complex. Furthermore, we find that the nephrocyte diaphragm is completely lost in flies lacking the orthologues of nephrin or NEPH1-a phenotype resembling loss of the slit diaphragm in the absence of either nephrin (as in human congenital nephrotic syndrome of the Finnish type, NPHS1) or NEPH1. These changes markedly impair filtration function in the nephrocyte. The similarities we describe between invertebrate nephrocytes and vertebrate podocytes provide evidence suggesting that the two cell types are evolutionarily related, and establish the nephrocyte as a simple model in which to study podocyte biology and podocyte-associated diseases.
Figures


References
-
- Smith HW. From Fish to Philosopher. Little, Brown; Boston: 1953.
-
- Ruppert EE. Evolutionary Origin of the Vertebrate Nepron. American Zoologist. 1994;34:542–533.
-
- Patrakka J, et al. Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int. 2000;58:972–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

