A protein that replaces the entire cellular eIF4F complex
- PMID: 18971945
- PMCID: PMC2599872
- DOI: 10.1038/emboj.2008.228
A protein that replaces the entire cellular eIF4F complex
Abstract
The eIF4F cap-binding complex mediates the initiation of cellular mRNA translation. eIF4F is composed of eIF4E, which binds to the mRNA cap, eIF4G, which indirectly links the mRNA cap with the 43S pre-initiation complex, and eIF4A, which is a helicase necessary for initiation. Viral nucleocapsid proteins (N) function in both genome replication and RNA encapsidation. Surprisingly, we find that hantavirus N has multiple intrinsic activities that mimic and substitute for each of the three peptides of the cap-binding complex thereby enhancing the translation of viral mRNA. N binds with high affinity to the mRNA cap replacing eIF4E. N binds directly to the 43S pre-initiation complex facilitating loading of ribosomes onto capped mRNA functionally replacing eIF4G. Finally, N obviates the requirement for the helicase, eIF4A. The expression of a multifaceted viral protein that functionally supplants the cellular cap-binding complex is a unique strategy for viral mRNA translation initiation. The ability of N to directly mediate translation initiation would ensure the efficient translation of viral mRNA.
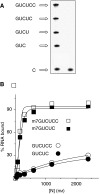
Figures
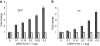




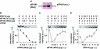
References
-
- Blakqori G, Kochs G, Haller O, Weber F (2003) Functional L polymerase of La Crosse virus allows in vivo reconstitution of recombinant nucleocapsids. J Gen Virol 84 (Part 5): 1207–1214 - PubMed
-
- Bochkov YA, Palmenberg AC (2006) Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques 41: 283–284, 286, 288 passim - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

