Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions
- PMID: 18972034
- PMCID: PMC2656348
- DOI: 10.1039/b813246a
Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions
Abstract
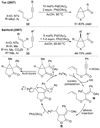
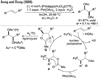
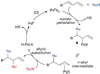
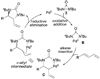
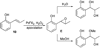
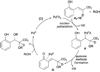
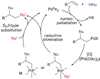
Alkene difunctionalization, the addition of two functional groups across a double bond, exemplifies a class of reactions with significant synthetic potential. This emerging area examines recent developments of palladium-catalyzed difunctionalization reactions, with a focus on mechanistic strategies that allow for functionalization of a common palladium alkyl intermediate.
Figures


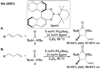

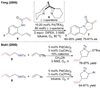
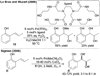
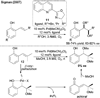
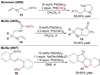
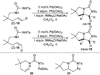
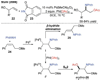
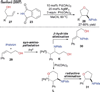

References
-
-
For a review, see Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547.
-
-
-
For a review, see Bäckvall J-E. Metal-Catalyzed Cross-Coupling Reactions. (2nd edn) 2004;vol. 2:479–529.
-
-
- Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308–7309. - PubMed
-
- Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762–763. - PubMed
-
- Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688–11689. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

