Imaging of hereditary hemorrhagic telangiectasia
- PMID: 18972161
- PMCID: PMC2705726
- DOI: 10.1007/s00270-008-9344-2
Imaging of hereditary hemorrhagic telangiectasia
Abstract
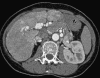
This pictorial review is based on our experience of the follow-up of 120 patients at our multidisciplinary center for hereditary hemorrhagic telangiectasia (HHT). Rendu-Osler-Weber disease or HHT is a multiorgan autosomal dominant disorder with high penetrance, characterized by epistaxis, mucocutaneous telangiectasis, and visceral arteriovenous malformations (AVMs). The research on gene mutations is fundamental and family screening by clinical examination, chest X-ray, research of pulmonary shunting, and abdominal color Doppler sonography is absolutely necessary. The angioarchitecture of pulmonary AVMs can be studied by unenhanced multidetector computed tomography; however, all other explorations of liver, digestive bowels, or brain require administration of contrast media. Magnetic resonance angiography is helpful for central nervous system screening, in particular for the spinal cord, but also for pulmonary, hepatic, and pelvic AVMs. Knowledge of the multiorgan involvement of HHT, mechanism of complications, and radiologic findings is fundamental for the correct management of these patients.
Figures

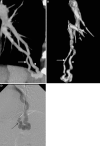
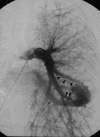
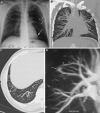

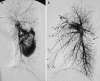
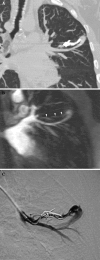

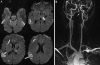
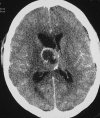


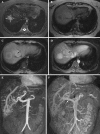
References
-
- Lesca G, Olivieri C, Burnichon N et al (2007) Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med 9:14–22 - PubMed
-
- White RI Jr, Lynch-Nyhan A, Terry P et al (1988) Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 169:663–669 - PubMed
-
- Remy J, Remy-Jardin M, Wattinne L, Deffontaines C (1992) Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology 182:809–816 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

