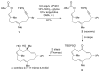
Nickel-catalyzed Negishi cross-couplings of secondary nucleophiles with secondary propargylic electrophiles at room temperature
- PMID: 18972493
- PMCID: PMC2790060
- DOI: 10.1002/anie.200802784
Nickel-catalyzed Negishi cross-couplings of secondary nucleophiles with secondary propargylic electrophiles at room temperature
Figures
Similar articles
-
Catalytic enantioselective cross-couplings of secondary alkyl electrophiles with secondary alkylmetal nucleophiles: Negishi reactions of racemic benzylic bromides with achiral alkylzinc reagents.J Am Chem Soc. 2012 Oct 17;134(41):17003-6. doi: 10.1021/ja308460z. Epub 2012 Oct 5. J Am Chem Soc. 2012. PMID: 23039358 Free PMC article.
-
Nickel-catalyzed cross-couplings of organosilicon reagents with unactivated secondary alkyl bromides.J Am Chem Soc. 2004 Jun 30;126(25):7788-9. doi: 10.1021/ja047433c. J Am Chem Soc. 2004. PMID: 15212521
-
Nickel-catalyzed cross-coupling reaction of alkyl halides with organozinc and Grignard reagents with 1,3,8,10-tetraenes as additives.Angew Chem Int Ed Engl. 2004 Nov 19;43(45):6180-2. doi: 10.1002/anie.200460246. Angew Chem Int Ed Engl. 2004. PMID: 15549748 No abstract available.
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
-
Trends in the Usage of Bidentate Phosphines as Ligands in Nickel Catalysis.Chem Rev. 2020 Jul 8;120(13):6124-6196. doi: 10.1021/acs.chemrev.9b00682. Epub 2020 Jun 3. Chem Rev. 2020. PMID: 32491839 Review.
Cited by
-
Synthesis of Secondary and Tertiary Alkylboranes via Formal Hydroboration of Terminal and 1,1-Disubstituted Alkenes.Org Lett. 2016 Nov 4;18(21):5760-5763. doi: 10.1021/acs.orglett.6b03090. Epub 2016 Oct 27. Org Lett. 2016. PMID: 27786484 Free PMC article.
-
Dialkyl Ether Formation by Nickel-Catalyzed Cross-Coupling of Acetals and Aryl Iodides.Angew Chem Int Ed Engl. 2015 Aug 17;54(34):9876-80. doi: 10.1002/anie.201503936. Epub 2015 Jul 15. Angew Chem Int Ed Engl. 2015. PMID: 26219537 Free PMC article.
-
Asymmetric synthesis via stereospecific C-N and C-O bond activation of alkyl amine and alcohol derivatives.Chem Commun (Camb). 2018 Oct 30;54(87):12286-12301. doi: 10.1039/c8cc07093h. Chem Commun (Camb). 2018. PMID: 30283929 Free PMC article.
-
Nickel-catalyzed Kumada cross-coupling reactions of tertiary alkylmagnesium halides and aryl bromides/triflates.J Am Chem Soc. 2011 Jun 8;133(22):8478-81. doi: 10.1021/ja202769t. Epub 2011 May 12. J Am Chem Soc. 2011. PMID: 21553878 Free PMC article.
-
Highly selective nickel-catalyzed gem-difluoropropargylation of unactivated alkylzinc reagents.Nat Commun. 2017 Nov 13;8(1):1460. doi: 10.1038/s41467-017-01540-1. Nat Commun. 2017. PMID: 29133781 Free PMC article.
References
-
-
For reviews, see: Frisch AC, Beller M. Angew Chem, Int Ed. 2005;44:674–688.Netherton MR, Fu GC. In: Topics in Organometallic Chemistry: Palladium in Organic Synthesis. Tsuji J, editor. Springer; New York: 2005. pp. 85–108.Netherton MR, Fu GC. Adv Synth Catal. 2004;346:1525–1532.
-
-
-
Ready has reported copper-catalyzed couplings of secondary α-chloroketones with secondary alkylzinc reagents. He postulates that, unlike typical palladium- and nickel-catalyzed cross-couplings, these reactions proceed without oxidative addition of the electrophile to the catalyst. See: Malosh CF, Ready JM. J Am Chem Soc. 2004;126:10240–10241.
-
-
-
For a review of the Negishi reaction, see: Negishi E-i, Hu Q, Huang Z, Wang G, Yin N. In: The Chemistry of Organozinc Compounds. Rappoport Z, Marek I, editors. Wiley; New York: 2006. Chapter 11.
-
-
-
For pioneering studies of nickel-catalyzed Negishi reactions of alkyl electrophiles, see: Devasagayaraj A, Stüdemann T, Knochel P. Angew Chem, Int Ed Engl. 1995;34:2723–2725.Giovannini R, Stüdemann T, Devasagayaraj A, Dussin G, Knochel P. J Org Chem. 1999;64:3544–3553.
-
-
-
For mechanistic studies of nickel-catalyzed Negishi reactions of alkyl electrophiles (including leading references), see: Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J Am Chem Soc. 2006;128:13175–13183.. See also: Anderson TJ, Jones GD, Vicic DA. J Am Chem Soc. 2004;126:8100–8101.Lin X, Phillips DL. J Org Chem. 2008;73:3680–3688.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources