Hydrogen-bond detection, configuration assignment and rotamer correction of side-chain amides in large proteins by NMR spectroscopy through protium/deuterium isotope effects
- PMID: 18973166
- PMCID: PMC3018730
- DOI: 10.1002/cbic.200800467
Hydrogen-bond detection, configuration assignment and rotamer correction of side-chain amides in large proteins by NMR spectroscopy through protium/deuterium isotope effects
Abstract
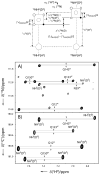
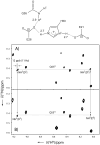
The configuration and hydrogen-bonding network of side-chain amides in a 35 kDa protein were determined by measuring differential and trans-hydrogen-bond H/D isotope effects by using the isotopomer-selective (IS)-TROSY technique, which leads to a reliable recognition and correction of erroneous rotamers that are frequently found in protein structures. First, the differential two-bond isotope effects on carbonyl (13)C' shifts, which are defined as Delta(2)Delta(13)C'(ND) = (2)Delta(13)C'(ND(E))-(2)Delta(13)C'(ND(Z)), provide a reliable means for the configuration assignment for side-chain amides, because environmental effects (hydrogen bonds and charges, etc.) are greatly attenuated over the two bonds that separate the carbon and hydrogen atoms, and the isotope effects fall into a narrow range of positive values. Second and more importantly, the significant variations in the differential one-bond isotope effects on (15)N chemical shifts, which are defined as Delta(1)Delta(15)N(D) = (1)Delta(15)N(D(E))-(1)Delta(15)N(D(Z)) can be correlated with hydrogen-bonding interactions, particularly those involving charged acceptors. The differential one-bond isotope effects are additive, with major contributions from intrinsic differential conjugative interactions between the E and Z configurations, H-bonding interactions, and charge effects. Furthermore, the pattern of trans-H-bond H/D isotope effects can be mapped onto more complicated hydrogen-bonding networks that involve bifurcated hydrogen-bonds. Third, the correlations between Delta(1)Delta(15)N(D) and hydrogen-bonding interactions afford an effective means for the correction of erroneous rotamer assignments of side-chain amides. Rotamer correction by differential isotope effects is not only robust, but also simple and can be applied to large proteins.
Figures




References
-
- Creighton TE. Proteins: Structure and Molecular Properties. 2. Freeman, W. H. and Company; New York: 1993.
-
- Word JM, Lovell SC, Richardson JS, Richardson DC. J Mol Biol. 1999;285:1735–1747. - PubMed
-
- Weichenberger CX, Sippl MJ. Bioinformatics. 2006;22:1397–1398. - PubMed
-
- Hansen PE. Magn Reson Chem. 2000;38:1–10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

