Severe deficiency of coagulation Factor VII results in spontaneous cardiac fibrosis in mice
- PMID: 18973189
- PMCID: PMC4269473
- DOI: 10.1002/path.2454
Severe deficiency of coagulation Factor VII results in spontaneous cardiac fibrosis in mice
Abstract
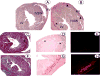
Mice genetically modified to produce low levels (approximately 1% of wild-type) of coagulation FVII presented with echocardiographic evidence of heart abnormalities. Decreases in ventricular size and reductions in systolic and diastolic functions were found, suggestive of a restrictive cardiomyopathy and consistent with an infiltrative myopathic process. Microscopic analysis of mouse hearts showed severe patchy fibrosis in the low-FVII mice. Haemosiderin deposition was discovered in hearts of these mice, along with increases in inflammatory cell number, ultimately resulting in widespread collagen deposition. Significant increases in mRNA levels of TGFbeta, TNFalpha and several matrix metalloproteinases in low-FVII mice, beginning at early ages, supported a state of cardiac remodelling associated with the fibrotic pathology. Mechanistic time-course studies suggested that cardiac fibrosis in low-FVII mice originated from bleeding in heart tissue, resulting in the recruitment of leukocytes, which released inflammatory mediators and induced collagen synthesis and secretion. These events led to necrosis of cardiomyocytes and collagen deposition, characteristics of cardiac fibrosis. The results of this study demonstrated that haemorrhagic and inflammatory responses to a severe FVII deficiency resulted in the development of cardiac fibrosis, observed echocardiographically as a restrictive cardiomyopathy, with compromised ventricular diastolic and systolic functions.
Conflict of interest statement
Conflict of Interest: None for any author
Figures






Similar articles
-
Male-specific cardiac pathologies in mice lacking either the A or B subunit of factor XIII.Thromb Haemost. 2008 Feb;99(2):401-8. doi: 10.1160/TH07-10-0599. Thromb Haemost. 2008. PMID: 18278192
-
Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15333-8. doi: 10.1073/pnas.242501899. Epub 2002 Nov 8. Proc Natl Acad Sci U S A. 2002. PMID: 12426405 Free PMC article.
-
IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H76-85. doi: 10.1152/ajpheart.01285.2008. Epub 2009 May 8. Am J Physiol Heart Circ Physiol. 2009. PMID: 19429811 Free PMC article.
-
An overview of inherited factor VII deficiency.Transfus Apher Sci. 2019 Oct;58(5):569-571. doi: 10.1016/j.transci.2019.08.006. Epub 2019 Aug 6. Transfus Apher Sci. 2019. PMID: 31447397 Review.
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Plasminogen activator inhibitor-1 (PAI-1) is cardioprotective in mice by maintaining microvascular integrity and cardiac architecture.Blood. 2010 Mar 11;115(10):2038-47. doi: 10.1182/blood-2009-09-244962. Epub 2009 Dec 15. Blood. 2010. PMID: 20009036 Free PMC article.
-
Differential roles of factors IX and XI in murine placenta and hemostasis under conditions of low tissue factor.Blood Adv. 2020 Jan 14;4(1):207-216. doi: 10.1182/bloodadvances.2019000921. Blood Adv. 2020. PMID: 31935292 Free PMC article.
-
Straight to the heart: Pleiotropic antiarrhythmic actions of oral anticoagulants.Pharmacol Res. 2019 Jul;145:104257. doi: 10.1016/j.phrs.2019.104257. Epub 2019 May 2. Pharmacol Res. 2019. PMID: 31054953 Free PMC article. Review.
-
Tissue factor, protease activated receptors and pathologic heart remodelling.Thromb Haemost. 2014 Nov;112(5):893-900. doi: 10.1160/TH14-03-0243. Epub 2014 Aug 7. Thromb Haemost. 2014. PMID: 25104210 Free PMC article. Review.
-
The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is "Inflammation" Always Inflammation?Mediators Inflamm. 2016;2016:2856213. doi: 10.1155/2016/2856213. Epub 2016 Aug 11. Mediators Inflamm. 2016. PMID: 27597803 Free PMC article. Review.
References
-
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, et al. Role of Tissue Factor in embryonic blood vessel development. Nature. 1996;383:73–75. - PubMed
-
- Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. - PubMed
-
- Rosen E, Chan JCY, Idusogie E, Clotman F, Vlasuk G, Luther T, et al. Mice lacking Factor VII develop normally but suffer fatal perinatal bleeding. Nature. 1997;390:290–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

