Mechanisms of alpha-defensin bactericidal action: comparative membrane disruption by Cryptdin-4 and its disulfide-null analogue
- PMID: 18973303
- PMCID: PMC2645939
- DOI: 10.1021/bi800335e
Mechanisms of alpha-defensin bactericidal action: comparative membrane disruption by Cryptdin-4 and its disulfide-null analogue
Abstract
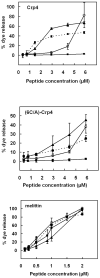
Mammalian alpha-defensins all have a conserved triple-stranded beta-sheet structure that is constrained by an invariant tridisulfide array, and the peptides exert bactericidal effects by permeabilizing the target cell envelope. Curiously, the disordered, disulfide-null variant of mouse alpha-defensin cryptdin-4 (Crp4), termed (6C/A)-Crp4, has bactericidal activity equal to or greater than that of the native peptide, providing a rationale for comparing the mechanisms by which the peptides interact with and disrupt phospholipid vesicles of defined composition. For both live Escherichia coli ML35 cells and model membranes, disordered (6C/A)-Crp4 induced leakage in a manner similar to that of Crp4 but had less overall membrane permeabilizing activity. Crp4 induction of the leakage of the fluorophore from electronegative liposomes was strongly dependent on vesicle lipid charge and composition, and the incorporation of cardiolipin into liposomes of low electronegative charge to mimic bacterial membrane composition conferred sensitivity to Crp4- and (6C/A)-Crp4-mediated vesicle lysis. Membrane perturbation studies using biomimetic lipid/polydiacetylene vesicles showed that Crp4 inserts more pronouncedly into membranes containing a high fraction of electronegative lipids or cardiolipin than (6C/A)-Crp4 does, correlating directly with measurements of induced leakage. Fluorescence resonance energy transfer experiments provided evidence that Crp4 translocates across highly charged or cardiolipin-containing membranes, in a process coupled with membrane permeabilization, but (6C/A)-Crp4 did not translocate across lipid bilayers and consistently displayed membrane surface association. Thus, despite the greater in vitro bactericidal activity of (6C/A)-Crp4, native, beta-sheet-containing Crp4 induces membrane permeabilization more effectively than disulfide-null Crp4 by translocating and forming transient membrane defects. (6C/A)-Crp4, on the other hand, appears to induce greater membrane disintegration.
Figures







Similar articles
-
Quantitative interactions between cryptdin-4 amino terminal variants and membranes.Peptides. 2003 Nov;24(11):1795-805. doi: 10.1016/j.peptides.2003.08.020. Peptides. 2003. PMID: 15019212
-
Interactions of mouse Paneth cell alpha-defensins and alpha-defensin precursors with membranes. Prosegment inhibition of peptide association with biomimetic membranes.J Biol Chem. 2003 Apr 18;278(16):13838-46. doi: 10.1074/jbc.M212115200. Epub 2003 Feb 6. J Biol Chem. 2003. PMID: 12574157
-
Functional analysis of the alpha-defensin disulfide array in mouse cryptdin-4.J Biol Chem. 2004 Oct 15;279(42):44188-96. doi: 10.1074/jbc.M406154200. Epub 2004 Aug 5. J Biol Chem. 2004. PMID: 15297466
-
Anionic amino acids near the pro-alpha-defensin N terminus mediate inhibition of bactericidal activity in mouse pro-cryptdin-4.J Biol Chem. 2009 Mar 13;284(11):6826-31. doi: 10.1074/jbc.M807024200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106102 Free PMC article.
-
Kinetics of cryptdin-4 translocation coupled with peptide-induced vesicle leakage.Biochemistry. 2007 Oct 23;46(42):11882-91. doi: 10.1021/bi701110m. Epub 2007 Oct 2. Biochemistry. 2007. PMID: 17910476
Cited by
-
Therapeutic compounds targeting Lipid II for antibacterial purposes.Infect Drug Resist. 2019 Aug 23;12:2613-2625. doi: 10.2147/IDR.S215070. eCollection 2019. Infect Drug Resist. 2019. PMID: 31692545 Free PMC article.
-
Paneth cell α-defensins in enteric innate immunity.Cell Mol Life Sci. 2011 Jul;68(13):2215-29. doi: 10.1007/s00018-011-0714-6. Epub 2011 May 11. Cell Mol Life Sci. 2011. PMID: 21560070 Free PMC article. Review.
-
A Requirement for Metamorphic Interconversion in the Antimicrobial Activity of Chemokine XCL1.Biochemistry. 2016 Jul 12;55(27):3784-93. doi: 10.1021/acs.biochem.6b00353. Epub 2016 Jun 28. Biochemistry. 2016. PMID: 27305837 Free PMC article.
-
The α-defensin salt-bridge induces backbone stability to facilitate folding and confer proteolytic resistance.Amino Acids. 2012 Oct;43(4):1471-83. doi: 10.1007/s00726-012-1220-3. Amino Acids. 2012. PMID: 22286872 Free PMC article.
-
Molecular diversity of antimicrobial effectors in the oyster Crassostrea gigas.BMC Evol Biol. 2010 Jan 25;10:23. doi: 10.1186/1471-2148-10-23. BMC Evol Biol. 2010. PMID: 20100329 Free PMC article.
References
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000;1:113–118. - PubMed
-
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. - PubMed
-
- Jing W, Hunter HN, Tanabe H, Ouellette AJ, Vogel HJ. Solution structure of cryptdin-4, a mouse paneth cell alpha-defensin. Biochemistry. 2004;43:15759–15766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

