Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide
- PMID: 18973305
- PMCID: PMC4733869
- DOI: 10.1021/bi8005384
Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide
Abstract
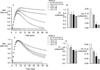
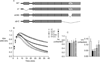
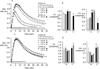
Elastin is the polymeric protein responsible for the properties of extensibility and elastic recoil of the extracellular matrix in a variety of tissues. Although proper assembly of the elastic matrix is crucial for its durability, the process by which this assembly takes place is not well-understood. Recent data suggest the complex interaction of tropoelastin, the monomeric form of elastin, with a number of other elastic matrix-associated proteins, including fibrillins, fibulins, and matrix-associated glycoprotein (MAGP), is important to achieve the proper architecture of the elastic matrix. At the same time, it is becoming clear that self-assembly properties intrinsic to tropoelastin itself, reflected in a temperature-induced phase separation known as coacervation, are also important in this assembly process. In this study, using a well-characterized elastin-like polypeptide that mimics the self-assembly properties of full-length tropoelastin, the process of self-assembly is deconstructed into "coacervation" and "maturation" stages that can be distinguished kinetically by different parameters. Members of the fibrillin, fibulin, and MAGP families of proteins are shown to profoundly affect both the kinetics of self-assembly and the morphology of the maturing coacervate, restricting the growth of coacervate droplets and, in some cases, causing clustering of droplets into fibrillar structures.
Figures









References
-
- Mithieux SM, Weiss AS. Elastin. Adv. Protein Chem. 2005;70:437–461. - PubMed
-
- Brown-Augsburger P, Broekelmann T, Mecham L, Mercer R, Gibson MA, Cleary EG, Abrams WR, Rosenbloom J, Mecham RP. Microfibril-associated glycoprotein binds to the carboxyl-terminal domain of tropoelastin and is a substrate for transglutaminase. J. Biol. Chem. 1994;269:28443–28449. - PubMed
-
- Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP. Interaction of tro-poelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J. Biol. Chem. 2000;275:24400–24406. - PubMed
-
- Jensen SA, Reinhardt DP, Gibson MA, Weiss AS. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J. Biol. Chem. 2001;276:39661–39616. - PubMed
-
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo . Nature. 2002;415:171–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

