Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development
- PMID: 18973570
- PMCID: PMC2675014
- DOI: 10.1111/j.1460-9568.2008.06475.x
Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development
Abstract
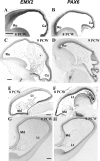
The transcription factors Emx2 and Pax6 are expressed in the proliferating zones of the developing rodent neocortex, and gradients of expression interact in specifying caudal and rostral identities. Pax6 is also involved in corticoneurogenesis, being expressed by radial glial progenitors that give rise to cells that also sequentially express Tbr2, NeuroD and Tbr1, genes temporally downstream of Pax6. In this study, using in situ hybridization, we analysed the expression of EMX2, PAX6, TBR2, NEUROD and TBR1 mRNA in the developing human cortex between 8 and 12 postconceptional weeks (PCW). EMX2 mRNA was expressed in the ventricular (VZ) and subventricular zones (SVZ), but also in the cortical plate, unlike in the rodent. However, gradients of expression were similar to that of the rodent at all ages studied. PAX6 mRNA expression was limited to the VZ and SVZ. At 8 PCW, PAX6 was highly expressed rostrally but less so caudally, as has been seen in the rodent, however this gradient disappeared early in corticogenesis, by 9 PCW. There was less restricted compartment-specific expression of TBR2, NEUROD and TBR1 mRNA than in the rodent, where the gradients of expression were similar to that of PAX6 prior to 9 PCW. The gradient disappeared for TBR2 by 10 PCW, and for NEUROD and TBR1 by 12 PCW. These data support recent reports that EMX2 but not PAX6 is more directly involved in arealization, highlighting that analysis of human development allows better spatio-temporal resolution than studies in rodents.
Figures




References
-
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. - PubMed
-
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J. Comp. Neurol. 2003;457:345–360. - PubMed
-
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JLR. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

