Effects of general anesthetics on visceral pain transmission in the spinal cord
- PMID: 18973669
- PMCID: PMC2584043
- DOI: 10.1186/1744-8069-4-50
Effects of general anesthetics on visceral pain transmission in the spinal cord
Abstract
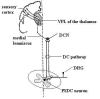
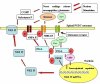
Current evidence suggests an analgesic role for the spinal cord action of general anesthetics; however, the cellular population and intracellular mechanisms underlying anti-visceral pain by general anesthetics still remain unclear. It is known that visceral nociceptive signals are transmited via post-synaptic dorsal column (PSDC) and spinothalamic tract (STT) neuronal pathways and that the PSDC pathway plays a major role in visceral nociception. Animal studies report that persistent changes including nociception-associated molecular expression (e.g. neurokinin-1 (NK-1) receptors) and activation of signal transduction cascades (such as the protein kinase A [PKA]-c-AMP-responsive element binding [CREB] cascade)-in spinal PSDC neurons are observed following visceral pain stimulation. The clinical practice of interruption of the spinal PSDC pathway in patients with cancer pain further supports a role of this group of neurons in the development and maintenance of visceral pain. We propose the hypothesis that general anesthetics might affect critical molecular targets such as NK-1 and glutamate receptors, as well as intracellular signaling by CaM kinase II, protein kinase C (PKC), PKA, and MAP kinase cascades in PSDC neurons, which contribute to the neurotransmission of visceral pain signaling. This would help elucidate the mechanism of antivisceral nociception by general anesthetics at the cellular and molecular levels and aid in development of novel therapeutic strategies to improve clinical management of visceral pain.
Figures
Similar articles
-
The role of c-AMP-dependent protein kinase in spinal cord and post synaptic dorsal column neurons in a rat model of visceral pain.Neurochem Int. 2007 Apr;50(5):710-8. doi: 10.1016/j.neuint.2007.01.006. Epub 2007 Jan 25. Neurochem Int. 2007. PMID: 17320244 Free PMC article.
-
NK-1-receptor-mediated lesion of spinal post-synaptic dorsal column neurons might improve intractable visceral pain of cancer origin.Med Hypotheses. 2011 Jan;76(1):102-4. doi: 10.1016/j.mehy.2010.08.042. Epub 2010 Sep 9. Med Hypotheses. 2011. PMID: 20826067
-
Activation of ERK signaling in rostral ventromedial medulla is dependent on afferent input from dorsal column pathway and contributes to acetic acid-induced visceral nociception.Neurochem Int. 2013 Nov;63(5):389-96. doi: 10.1016/j.neuint.2013.07.005. Epub 2013 Jul 19. Neurochem Int. 2013. PMID: 23876632
-
The role of dorsal columns pathway in visceral pain.Physiol Res. 2004;53 Suppl 1:S125-30. Physiol Res. 2004. PMID: 15119943 Review.
-
Pharmacology of visceral pain: central factors.Dig Dis. 2009;27 Suppl 1:31-41. doi: 10.1159/000268119. Epub 2010 Mar 4. Dig Dis. 2009. PMID: 20203495 Review.
Cited by
-
Drug management of visceral pain: concepts from basic research.Pain Res Treat. 2012;2012:265605. doi: 10.1155/2012/265605. Epub 2012 Apr 24. Pain Res Treat. 2012. PMID: 22619712 Free PMC article.
-
Skin, fascias, and scars: symptoms and systemic connections.J Multidiscip Healthc. 2013 Dec 28;7:11-24. doi: 10.2147/JMDH.S52870. eCollection 2013. J Multidiscip Healthc. 2013. PMID: 24403836 Free PMC article.
-
Effects of intrathecal bupivacaine on the NR2B/CaMKIIα/CREB signaling pathway in the rat lumbar spinal cord.Mol Med Rep. 2018 Mar;17(3):4508-4514. doi: 10.3892/mmr.2018.8448. Epub 2018 Jan 17. Mol Med Rep. 2018. PMID: 29344649 Free PMC article.
-
Differential roles of phosphorylated AMPA receptor GluR1 subunits at Serine-831 and Serine-845 sites in spinal cord dorsal horn in a rat model of post-operative pain.Neurochem Res. 2011 Jan;36(1):170-6. doi: 10.1007/s11064-010-0288-y. Epub 2010 Oct 17. Neurochem Res. 2011. PMID: 20953906
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

