Abnormal spatial diffusion of Ca2+ in F508del-CFTR airway epithelial cells
- PMID: 18973672
- PMCID: PMC2584091
- DOI: 10.1186/1465-9921-9-70
Abnormal spatial diffusion of Ca2+ in F508del-CFTR airway epithelial cells
Abstract
Background: In airway epithelial cells, calcium mobilization can be elicited by selective autocrine and/or paracrine activation of apical or basolateral membrane heterotrimeric G protein-coupled receptors linked to phospholipase C (PLC) stimulation, which generates inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG) and induces Ca2+ release from endoplasmic reticulum (ER) stores.
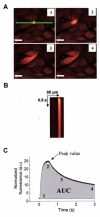
Methods: In the present study, we monitored the cytosolic Ca2+ transients using the UV light photolysis technique to uncage caged Ca2+ or caged IP3 into the cytosol of loaded airway epithelial cells of cystic fibrosis (CF) and non-CF origin. We compared in these cells the types of Ca2+ receptors present in the ER, and measured their Ca2+ dependent activity before and after correction of F508del-CFTR abnormal trafficking either by low temperature or by the pharmacological corrector miglustat (N-butyldeoxynojirimycin).
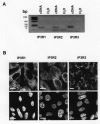
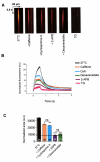
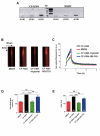
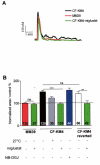
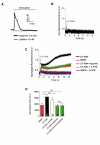
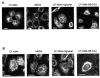
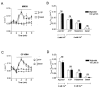
Results: We showed reduction of the inositol 1,4,5-trisphosphate receptors (IP3R) dependent-Ca2+ response following both correcting treatments compared to uncorrected cells in such a way that Ca2+ responses (CF+treatment vs wild-type cells) were normalized. This normalization of the Ca2+ rate does not affect the activity of Ca2+-dependent chloride channel in miglustat-treated CF cells. Using two inhibitors of IP3R1, we observed a decrease of the implication of IP3R1 in the Ca2+ response in CF corrected cells. We observed a similar Ca2+ mobilization between CF-KM4 cells and CFTR-cDNA transfected CF cells (CF-KM4-reverted). When we restored the F508del-CFTR trafficking in CFTR-reverted cells, the specific IP3R activity was also reduced to a similar level as in non CF cells. At the structural level, the ER morphology of CF cells was highly condensed around the nucleus while in non CF cells or corrected CF cells the ER was extended at the totality of cell.
Conclusion: These results suggest reversal of the IP3R dysfunction in F508del-CFTR epithelial cells by correction of the abnormal trafficking of F508del-CFTR in cystic fibrosis cells. Moreover, using CFTR cDNA-transfected CF cells, we demonstrated that abnormal increase of IP3R Ca2+ release in CF human epithelial cells could be the consequence of F508del-CFTR retention in ER compartment.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

