Regulation of vascular permeability by sphingosine 1-phosphate
- PMID: 18973762
- PMCID: PMC2693384
- DOI: 10.1016/j.mvr.2008.09.005
Regulation of vascular permeability by sphingosine 1-phosphate
Abstract
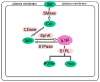
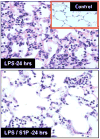
A significant and sustained increase in vascular permeability is a hallmark of acute inflammatory diseases such as acute lung injury (ALI) and sepsis and is an essential component of tumor metastasis, angiogenesis, and atherosclerosis. Sphingosine 1-phosphate (S1P), an endogenous bioactive lipid produced in many cell types, regulates endothelial barrier function by activation of its G-protein coupled receptor S1P(1). S1P enhances vascular barrier function through a series of profound events initiated by S1P(1) ligation with subsequent downstream activation of the Rho family of small GTPases, cytoskeletal reorganization, adherens junction and tight junction assembly, and focal adhesion formation. Furthermore, recent studies have identified transactivation of S1P(1) signaling by other barrier-enhancing agents as a common mechanism for promoting endothelial barrier function. This review summarizes the state of our current knowledge about the mechanisms through which the S1P/S1P(1) axis reduces vascular permeability, which remains an area of active investigation that will hopefully produce novel therapeutic agents in the near future.
Figures

Similar articles
-
In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights.Cell Signal. 2005 Feb;17(2):131-9. doi: 10.1016/j.cellsig.2004.08.006. Cell Signal. 2005. PMID: 15494205 Review.
-
Role of FAK in S1P-regulated endothelial permeability.Microvasc Res. 2012 Jan;83(1):22-30. doi: 10.1016/j.mvr.2011.08.012. Epub 2011 Sep 5. Microvasc Res. 2012. PMID: 21925517 Free PMC article. Review.
-
Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury.Am J Respir Cell Mol Biol. 2013 Jul;49(1):6-17. doi: 10.1165/rcmb.2012-0411TR. Am J Respir Cell Mol Biol. 2013. PMID: 23449739 Free PMC article. Review.
-
Endothelial cell barrier regulation by sphingosine 1-phosphate.J Cell Biochem. 2004 Aug 15;92(6):1075-85. doi: 10.1002/jcb.20088. J Cell Biochem. 2004. PMID: 15258893 Review.
-
Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature.Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H33-42. doi: 10.1152/ajpheart.00097.2008. Epub 2008 Nov 14. Am J Physiol Heart Circ Physiol. 2009. PMID: 19011048 Free PMC article.
Cited by
-
Heterogeneous elastic response of human lung microvascular endothelial cells to barrier modulating stimuli.Nanomedicine. 2013 Oct;9(7):875-84. doi: 10.1016/j.nano.2013.03.006. Epub 2013 Mar 20. Nanomedicine. 2013. PMID: 23523769 Free PMC article.
-
Sphingosine-1-Phosphate Receptor Modulators and Oligodendroglial Cells: Beyond Immunomodulation.Int J Mol Sci. 2020 Oct 13;21(20):7537. doi: 10.3390/ijms21207537. Int J Mol Sci. 2020. PMID: 33066042 Free PMC article. Review.
-
Tonic regulation of vascular permeability.Acta Physiol (Oxf). 2013 Apr;207(4):628-49. doi: 10.1111/apha.12076. Epub 2013 Feb 25. Acta Physiol (Oxf). 2013. PMID: 23374222 Free PMC article. Review.
-
Sphingolipids, mycobacteria and host: Unraveling the tug of war.Front Immunol. 2022 Sep 15;13:1003384. doi: 10.3389/fimmu.2022.1003384. eCollection 2022. Front Immunol. 2022. PMID: 36189241 Free PMC article. No abstract available.
-
Dengue virus pathogenesis: an integrated view.Clin Microbiol Rev. 2009 Oct;22(4):564-81. doi: 10.1128/CMR.00035-09. Clin Microbiol Rev. 2009. PMID: 19822889 Free PMC article. Review.
References
-
- Andriopoulou P, et al. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–97. - PubMed
-
- Aschner JL, et al. Bradykinin- and thrombin-induced increases in endothelial permeability occur independently of phospholipase C but require protein kinase C activation. J Cell Physiol. 1997;173:387–96. - PubMed
-
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. - PubMed
-
- Bensadoun ES, et al. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:1819–28. - PubMed
-
- Broussard JA, et al. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

