Discovery of new biosynthetic pathways: the lipid A story
- PMID: 18974037
- PMCID: PMC2674688
- DOI: 10.1194/jlr.R800060-JLR200
Discovery of new biosynthetic pathways: the lipid A story
Abstract
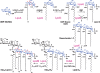
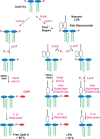
The outer monolayer of the outer membrane of Gram-negative bacteria consists of the lipid A component of lipopolysaccharide (LPS), a glucosamine-based saccharolipid that is assembled on the inner surface of the inner membrane. The first six enzymes of the lipid A pathway are required for bacterial growth and are excellent targets for the development of new antibiotics. Following assembly, the ABC transporter MsbA flips nascent LPS to the periplasmic side of the inner membrane, whereupon additional transport proteins direct it to the outer surface of the outer membrane. Depending on the bacterium, various covalent modifications of the lipid A moiety may occur during the transit of LPS to the outer membrane. These extra-cytoplasmic modification enzymes are therefore useful as reporters for monitoring LPS trafficking. Because of its conserved structure in diverse Gram-negative pathogens, lipid A is recognized as foreign by the TLR4/MD2 receptor of the mammalian innate immune system, resulting in rapid macrophage activation and robust cytokine production.
Figures



References
-
- Takayama K., N. Qureshi, P. Mascagni, M. A. Nashed, L. Anderson, and C. R. H. Raetz. 1983. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A: complete structure of a diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J. Biol. Chem. 258 7379–7385. - PubMed
-
- Nishijima M., and C. R. H. Raetz. 1979. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J. Biol. Chem. 254 7837–7844. - PubMed
-
- Raetz C. R. H. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59 129–170. - PubMed
-
- Rietschel E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zähringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8 217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

