FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells
- PMID: 18974115
- PMCID: PMC2597644
- DOI: 10.1158/0008-5472.CAN-08-1968
FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells
Abstract
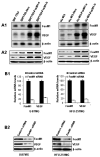
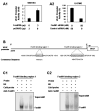
We previously found that FoxM1B is overexpressed in human glioblastomas and that forced FoxM1B expression in anaplastic astrocytoma cells leads to the formation of highly angiogenic glioblastoma in nude mice. However, the molecular mechanisms by which FoxM1B enhances glioma angiogenesis are currently unknown. In this study, we found that vascular endothelial growth factor (VEGF) is a direct transcriptional target of FoxM1B. FoxM1B overexpression increased VEGF expression, whereas blockade of FoxM1 expression suppressed VEGF expression in glioma cells. Transfection of FoxM1 into glioma cells directly activated the VEGF promoter, and inhibition of FoxM1 expression by FoxM1 siRNA suppressed VEGF promoter activation. We identified two FoxM1-binding sites in the VEGF promoter that specifically bound to the FoxM1 protein. Mutation of these FoxM1-binding sites significantly attenuated VEGF promoter activity. Furthermore, FoxM1 overexpression increased and inhibition of FoxM1 expression suppressed the angiogenic ability of glioma cells. Finally, an immunohistochemical analysis of 59 human glioblastoma specimens also showed a significant correlation between FoxM1 overexpression and elevated VEGF expression. Our findings provide both clinical and mechanistic evidence that FoxM1 contributes to glioma progression by enhancing VEGF gene transcription and thus tumor angiogenesis.
Figures



References
-
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255–68. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Feldkamp MM, Lau N, Rak J, Kerbel RS, Guha A. Normoxic and hypoxic regulation of vascular endothelial growth factor (VEGF) by astrocytoma cells is mediated by Ras. Int J Cancer. 1999;81:118–24. - PubMed
-
- Rahaman SO, Harbor PC, Chernova O, et al. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

