Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice
- PMID: 18974121
- PMCID: PMC2597356
- DOI: 10.1158/0008-5472.CAN-08-2135
Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice
Abstract
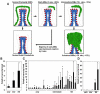
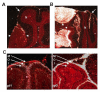
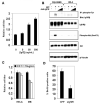
Medulloblastoma (MB) can arise in the cerebellum due to genetic activation of the Sonic Hedgehog (Shh) signaling pathway. During normal cerebellum development, Shh spurs the proliferation of granule neuron precursors (GNP), the precursor cells of MB. Mutations in the Shh receptor gene patched1 (ptc1+/-) lead to increased MB incidence in humans and mice. MB tumorigenesis in mice heterozygous for ptc1+/- shows distinct steps of progression. Most ptc1+/- mice form clusters of preneoplastic cells on the surface of the mature cerebellum that actively transcribe Shh target genes. In approximately 15% of mice, these preneoplastic cells will become fast-growing, lethal tumors. It was previously shown that the loss of function of insulin-like growth factor 2 (igf2) suppresses MB formation in ptc1+/- mice. We found that igf2 is not expressed in preneoplastic lesions but is induced as these lesions progress to more advanced MB tumors. Igf2 is not required for formation of preneoplastic lesions but is necessary for progression to advanced tumors. Exogenous Igf2 protein promoted proliferation of MB precursor cells (GNP) and a MB cell line, PZp53(MED). Blocking igf2 signaling inhibited growth of PZp53(MED) cells, implicating igf2 as a potential clinical target.
Figures


References
-
- Hahn H, Wojnowski L, Specht K, et al. Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem. 2000;275:28341–4. - PubMed
-
- Goussia AC, Bruner JM, Kyritsis AP, Agnantis NJ, Fuller GN. Cytogenetic and molecular genetic abnormalities in primitive neuroectodermal tumors of the central nervous system. Anticancer Res. 2000;20:65–73. - PubMed
-
- Raffel C, Jenkins RB, Frederick L, et al. Sporadic medulloblstomas contain PTCH mutations. Cancer Res. 1997;57:842–5. - PubMed
-
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. - PubMed
-
- Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila patched gene in positional signaling. Nature. 1991;353:184–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

